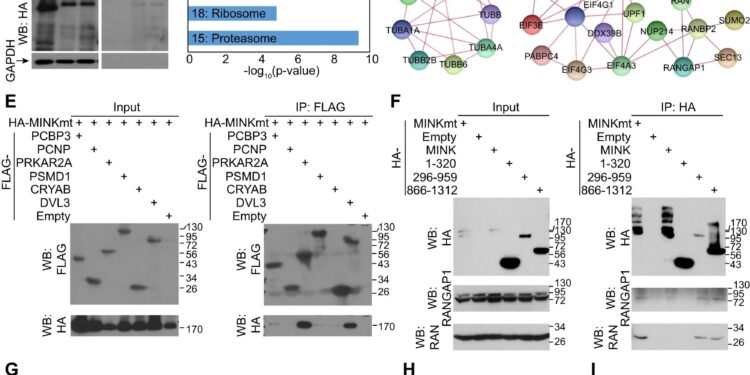
The MAP4K interactome and the effect on RANGAP1 subcellular distribution. A Western blot analysis of proteins after proximity labeling in ALS1-hiMNs at 10 dpi. B Venn diagram of proteins from two batches of samples analyzed by mass spectrometry. With an enrichment threshold of 2.5 times, 443 proteins were common to these two datasets, including proteins with no expression in the control group (0 in ctrl), proteins with changes of more than 2.5 times and proteins with changes of more than 10 times. . VS The 5 best KEGG routes. D STRING analysis of protein association networks in the 4 major KEGG pathways. E Validation of protein associations in HEK293T cells by co-immunoprecipitations (co-IP) and western blots. F co-IP assays in HEK293T cells showing association of MINK1mt with RANGAP1 or RAN. g Confocal images showing the subcellular distribution of RANGAP1 or RAN in ALS1-hiMNs at 52 dpi. Arrows indicate aggregated cytoplasmic RANGAP1 foci. Scale bar, 10 µm. H MINKmt enhances the nuclear/cytoplasmic (Nuc/Cyt) ratios of the indicated proteins in ALS1-hiMNs at 52 dpi (mean ± SEM; not= 30 neurons per group; ***p< 0.001, student t -test). I MINKmt enhances the soma size of ALS1-hiMNs at 52 dpi (mean ± SEM; not= 30 neurons per group; ***p < 0.001, student t-test). Credit: Cell death and disease(2024). DOI: 10.1038/s41419-023-06395-7
Researchers at UT Southwestern Medical Center have identified an investigational molecular compound that improved survival in cellular and mouse models of amyotrophic lateral sclerosis (ALS), the deadly neurodegenerative disease. Their conclusions, reported in Cell death and diseaseare promising for the potential development of treatments for ALS, for which there are no effective treatments.
“This study will significantly advance the field of ALS by providing a lead compound and signaling pathway for future research,” said study leader Chun-Li Zhang, Ph.D., professor of biology. molecular and biomedical sciences researcher WW Caruth, Jr. Research at UT Southwestern. Dr. Zhang is also a researcher at the Peter O’Donnell Jr. Brain Institute at UTSW.
ALS, also known as Lou Gehrig’s disease, affects hundreds of thousands of people worldwide. Beginning in midlife, ALS kills motor neurons over time, gradually robbing patients of the ability to walk, talk, swallow and breathe. Life expectancy is two to five years after diagnosis and has not changed despite decades of research, Dr. Zhang said.
Looking for potential therapies that could extend the lives of ALS patients, Dr. Zhang and colleagues tested compounds from a pharmaceutical library in a cellular model of ALS. Since it is impossible to sample motor neurons directly from ALS patients, previous studies have largely used neurons derived from pluripotent stem cells. However, Dr. Zhang said, these neurons were reset to an embryonic stage, losing age-related changes.
For the new study, the researchers used a different approach that converted ALS patients’ skin cells into motor neurons bearing marks of aging, providing a more realistic model. After dosing these cells with around 2,000 compounds, the researchers identified a promising one that they called Hit3.
This compound reversed some of the ALS-related morphological changes in cells, causing them to develop larger cell bodies, develop more complicated branches in their extensions, form more connections with muscle cells, and live much longer than cells that did not receive Hit3.
A closer look showed that Hit3 acts on cellular proteins called MAP4K, which play a key role in cells’ response to stress. Once activated, MAP4Ks regulate a cascade of other proteins involved in this role, a molecular pathway that appears essential in deciding whether motor neurons live or die and that has been implicated in ALS and other neurodegenerative diseases.
To determine what effect manipulating this pathway might have in animal models of ALS, the researchers gave mice with mutations in a gene called SOD1 – considered the most aggressive form of ALS – a Hit3-related compound called MAP4Ki. Mice that did not receive this compound suffered a dramatic loss of motor neurons and died on average after 129 days. However, mice that received MAP4Ki retained significantly more motor neurons and lived ten days longer.
“Even though MAP4Ki only prolonged survival by a small amount, our results suggest that treatments that block the MAP4K pathway may one day be therapeutically useful,” said Dr. Zhang.
Since MAP4Ki is not optimized for pharmaceutical use (it degrades quickly and cannot cross the blood-brain barrier, limiting its absorption), this compound has significant margin for chemical manipulations that could improve its business, Dr. Zhang said. Additionally, he noted, because targeting the MAP4K pathway has shown promise, researchers could eventually develop other drugs designed to act more successfully on this pathway. The hope is that this could potentially extend the lifespan of ALS patients.
More information:
Meng-Lu Liu et al, Aging-related human ALS motor neuron screens identify MAP4Ks as therapeutic targets for the disease, Cell death and disease(2024). DOI: 10.1038/s41419-023-06395-7
Provided by UT Southwestern Medical Center
Quote: An experimental compound extends life in a mouse model of amyotrophic lateral sclerosis (February 6, 2024) retrieved February 6, 2024 from
This document is subject to copyright. Apart from fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.