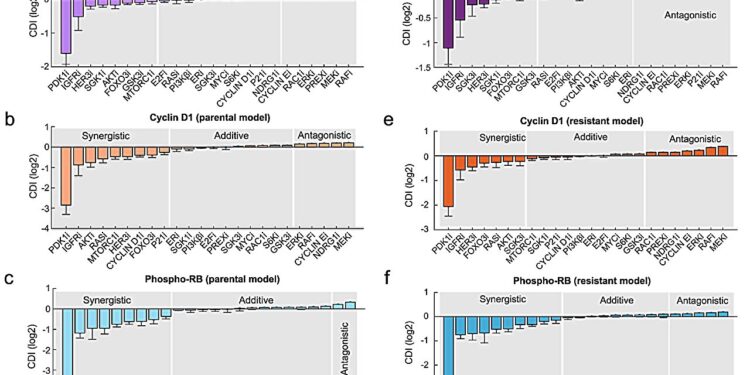
The computer model predicts synergistic combinations of drugs. a–f Drug synergy model prediction was generated using the drug interaction coefficient index (CDI) based on the levels of phospho-S6, cyclin D1, or phospho-Rb. Credit: npj Precision Oncology (2024). DOI: 10.1038/s41698-024-00496-y
Monash University-led research uses mathematics to predict how new combination therapies can help breast cancer patients who no longer respond to conventional therapies.
Published in npj Precision Oncology, the Monash Biomedicine Discovery Institute (BDI) study investigated breast cancer caused by a specific protein, PI3K, and how new combination therapies could effectively stop it. The study is titled “Integrative modeling uncovers p21-mediated drug resistance and prioritizes therapies for PIK3CA mutant breast cancer.” »
Associate Professor Lan Nguyen, co-senior author, said: “We created new computational models that mimic the behavior of the cancer-promoting protein PI3K and its broad downstream targets. This is essential because the PI3K pathway is mutated in approximately 30% of breast cancer cases. patients, and contributes to resistance to primary anticancer treatments.
“Using this mathematical approach, we predicted new combination therapies and confirmed through laboratory experiments that these new combination therapies are more effective in fighting PI3K mutant breast cancer cells than targeting PI3K alone.”
Dr Antonella Papa, co-senior author, said the study was an important step in understanding and overcoming drug resistance in breast cancer using innovative predictive models.
“Our study uncovered how breast cancer cells become resistant to alpelisib, a PI3K inhibitor used clinically for the treatment of PI3K mutant breast cancer,” she said. “Using this knowledge, we identified additional proteins that, when inhibited, restore sensitivity to alpelisib and stop the proliferation of resistant cells.”
Associate Professor Nguyen highlighted the formidable challenge of drug resistance in cancer treatment.
“Our study not only sheds light on the complex mechanisms underlying therapeutic resistance to alpelisib, but also provides a computational approach to systematically prioritize combination therapies in an unbiased manner,” he said. “This could accelerate the discovery of effective treatments, making it a valuable framework for future research in oncology and beyond.
“As drug resistance is a common cause of treatment failure, our research could lead to the testing and approval of new treatments that maintain their effectiveness for longer, potentially improving survival rates and quality of life for patients. patients. In the future, this could also mean fewer side effects and more personalized treatment options.
Associate Professor Nguyen said the next steps would involve rigorous preclinical evaluation of the identified drug combinations. “Following the success of preclinical studies, the initiation of clinical trials will be essential to confirm the safety and effectiveness of these new combination therapies in humans,” he said.
Dr Papa said: “Previous studies have demonstrated that similar treatments effectively reduce tumor growth using mice as a preclinical model. If preclinical validation progresses further, the first clinical trials could begin within a few years. Continued collaboration between us, researchers, clinicians and regulators will be essential to accelerate this process.
The authors acknowledged that the study was a team effort between two Monash BDI laboratories and that the collaborative nature of the work was instrumental in achieving these results. “This highlights the power of interdisciplinary approaches to address complex medical challenges like cancer,” they said.
More information:
Hon Yan Kelvin Yip et al, Integrative modeling uncovers p21-mediated drug resistance and prioritizes therapies for PIK3CA mutant breast cancer, npj Precision Oncology (2024). DOI: 10.1038/s41698-024-00496-y
Provided by Monash University
Quote: Innovative modeling can help breast cancer patients who don’t respond to treatment (February 5, 2024) retrieved February 5, 2024 from
This document is subject to copyright. Apart from fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.