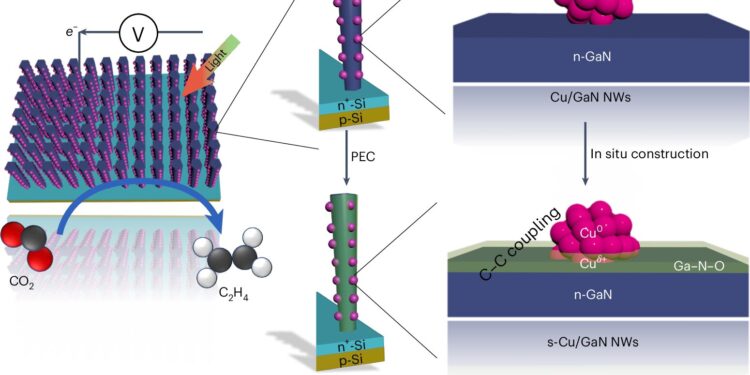
Schematic illustration of the in situ construction of the strongly coupled copper cluster and GaN NW/Si photocathode during CO PEC2RR process. Synthesis of nature (2024). DOI: 10.1038/s44160-024-00648-9
A key step towards the reuse of CO2 Making sustainable fuels requires stringing together carbon atoms, and an artificial photosynthesis system developed at the University of Michigan can link two of them into hydrocarbons with cutting-edge performance.
The system produces ethylene with much higher efficiency, yield and longevity than other artificial photosynthesis systems. Ethylene is a hydrocarbon commonly used in plastics. A direct application of the system would therefore be to recover carbon dioxide that would otherwise be released into the atmosphere to make plastics.
“The performance, or activity and stability, is about five to six times better than what is typically reported for solar energy or light-based carbon dioxide reduction to ethylene,” said Zetian Mi, a professor of electrical and computer engineering at the University of Michigan and corresponding author of the study. Synthesis of nature.
“Ethylene is actually the most widely produced organic compound in the world. But it is typically produced from oil and gas, under high temperatures and pressures, all of which emit CO2” . “
The long-term goal is to link longer chains of carbon and hydrogen atoms together to produce liquid fuels that can be easily transported. Part of the challenge is removing all the oxygen from the CO2 as a source of carbon and water, H2O, as a source of hydrogen.
The device absorbs light through two types of semiconductors: a forest of gallium nitride nanowires, each just 50 nanometers wide (a few hundred atoms), and the silicon base on which they were grown. The reaction that turns water and carbon dioxide into ethylene takes place on copper clusters, each about 30 atoms wide, that dot the nanowires.
The nanowires are immersed in carbon dioxide-enriched water and exposed to light equivalent to that of the sun at noon. The energy from the light releases electrons that split the water near the surface of the gallium nitride nanowires. This creates hydrogen that fuels the ethylene reaction but also oxygen that the gallium nitride absorbs to become gallium nitride oxide.
Copper is able to hold onto hydrogen and attach itself to the carbon in carbon dioxide, turning it into carbon monoxide. Using the hydrogen in the mixture and an injection of energy from light, the team believes that two molecules of carbon monoxide bond with the hydrogen. The reaction is thought to end at the interface between the copper and the gallium nitride oxide, where the two oxygen atoms are removed and replaced by three hydrogen atoms from the splitting of water.
The team found that 61 percent of the free electrons generated by the semiconductors using light contributed to the ethylene-producing reaction. Another catalyst made of silver and copper achieved a similar efficiency of about 50 percent, but it had to run in a carbon-based fluid and could only operate for a few hours before degrading. In contrast, the Michigan team’s device ran for 116 hours without slowing down, and the team ran similar devices for 3,000 hours.
This is partly due to the synergistic relationship between gallium nitride and the water splitting process: adding oxygen enhances the catalyst and allows for a self-healing process. The limits of the device’s longevity will be explored in future work.
Ultimately, the device produced ethylene at a rate more than four times that of the closest competing systems.
“In the future, we want to produce other multicarbon compounds such as three-carbon propanol or liquid products,” said Bingxing Zhang, a UM assistant research scientist in electrical and computer engineering and first author of the paper.
Liquid fuels, which could enable many existing transportation technologies to become sustainable, are Mi’s ultimate goal.
More information:
Zhang, B. et al., Interfacially coupled Cu/GaN cluster photocathode for efficient CO2 to the conversion of ethylene, Synthesis of nature (2024). DOI: 10.1038/s44160-024-00648-9
Provided by the University of Michigan
Quote:In a step toward solar fuels, a sustainable artificial photosynthesis facility chains two carbons together (2024, September 17) retrieved September 17, 2024 from
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without written permission. The content is provided for informational purposes only.