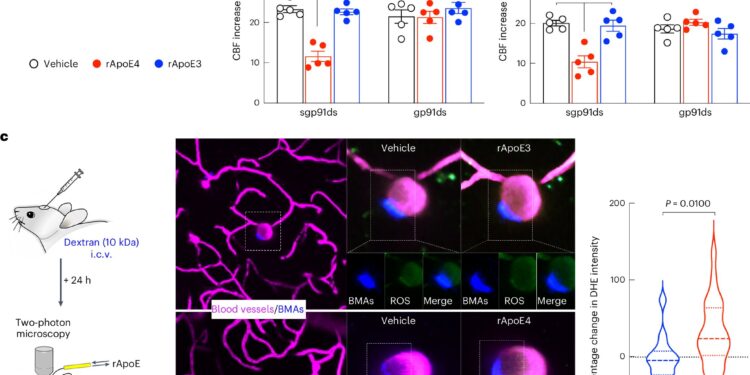
BAMs mediate the deleterious cerebrovascular effects of ApoE4 through NOX-derived ROS. Credit: Neuroscience of Nature (2024). DOI: 10.1038/s41593-024-01757-6
A new study helps explain why having ApoE4 – the genetic variant most closely linked to Alzheimer’s disease – increases the risk of neurodegeneration and white matter damage.
Researchers at Weill Cornell Medicine have discovered that immune cells in the brain called border-associated macrophages (BAMs) are a source of the protein ApoE4 and contribute to damage to blood vessels and brain tissue.
The study, published in Neuroscience of Naturecould help scientists identify new approaches to prevent or treat Alzheimer’s disease in people with the ApoE4 gene and other forms of age-related brain diseases.
The APOE gene encodes apolipoprotein E (ApoE), which plays many roles in the brain. There are also several common variants (ApoE2, ApoE3, and ApoE4), of which ApoE4 increases the risk of Alzheimer’s disease by up to 12 times. ApoE4 also increases the risk of white matter damage that leads to vascular dementia, the second most common cause of cognitive impairment after Alzheimer’s disease. However, how ApoE4 produces these harmful effects on the brain is not completely clear.
“Our study indicates that boundary-associated macrophages are a key mediator of these deleterious effects and helps us understand how ApoE4 may contribute to damage to blood vessels and white matter in the brain in patients with Alzheimer’s disease and other forms of age-related brain diseases,” said study co-senior author Laibaik Park, associate research professor of neuroscience in the Feil Family Brain and Mind Research Institute at Weill Cornell Medicine.
“We have already shown in another model that the beta-amyloid protein that accumulates in the brain of patients with Alzheimer’s disease interacts with a protein receptor for BAMs,” explains Antoine Anfray, professor of neuroscience at the Brain and Mind Research Institute and first author of the study. This triggers a chain reaction that damages blood vessels, preventing them from removing amyloid, leading to degeneration of brain tissue.
In their latest study, the researchers show that preclinical models genetically engineered to express the human ApoE4 variant developed impaired blood vessels and tissue damage in their brains, while those with the more benign ApoE3 remained healthy. They found that BAMs with the ApoE4 variant produce inflammatory oxygen free radicals, which damage blood vessels. As a result, the blood flow needed to remove waste and repair damage to brain tissue is limited.
Surprisingly, when animal models carrying the ApoE4 variant had their BAMs removed, they did not experience this cascade of damage. The study also showed that BAMs are not only the mediators of ApoE4-induced damage, but also the source of the ApoE4 that causes the damage. Therefore, reducing ApoE4 expression in BAMs eliminated the harmful vascular effects.
“These results show that BAMs are both the source and target of ApoE4 required for blood vessel damage,” said study senior author Costantino Iadecola, MD, director and president of the Brain and Mind Research Institute and the Anne Parrish Titzell Professor of Neurology at Weill Cornell Medicine.
The researchers also confirmed that ApoE4 and BAMs transferred to animal models that did not have the ApoE4 variant developed vascular and tissue damage. Alternatively, transplanting BAMs from animals carrying the ApoE3 variant to animals carrying the ApoE4 variant reversed the damage.
These findings may help explain why some patients are more likely to experience harmful swelling and bleeding in the brain when treated with amyloid-scavenging antibodies like lecanemab, a complication more common in patients with ApoE4. This complication, called an amyloid-related imaging abnormality (ARIA), requires stopping the treatment, limiting its benefit in slowing the progression of early-stage Alzheimer’s disease.
Understanding why blood vessels are more vulnerable in some patients could help scientists develop ways to prevent this adverse effect by suppressing ApoE4 production by BAMs. Iadecola and Park are working on developing such interventions, but they caution that more work is needed before the findings can be applied in the clinic.
For now, they are looking for ways to block the receptors that mediate ApoE4-related vascular damage to reduce or prevent the genetic variant’s harmful effects on the amyloid-beta clearance pathway.
“We now know that ApoE4 from border-associated macrophages increases blood vessel damage, but the next step would be to find a way to target macrophages to enhance amyloid and tau clearance,” Iadecola said. “Can genetic replacement of ApoE4 with the ApoE3 genetic variant more effectively clear amyloid accumulation? That will be a proof of concept.”
More information:
Anfray, A et al, A cell-autonomous role for border-associated macrophages in ApoE4 neurovascular dysfunction and susceptibility to white matter injury. Neuroscience of Nature (2024). DOI: 10.1038/s41593-024-01757-6. www.nature.com/articles/s41593-024-01757-6
Provided by Cornell University
Quote:Immune cells linked to blood vessel injury and neurodegeneration (2024, September 19) retrieved September 19, 2024 from
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without written permission. The content is provided for informational purposes only.