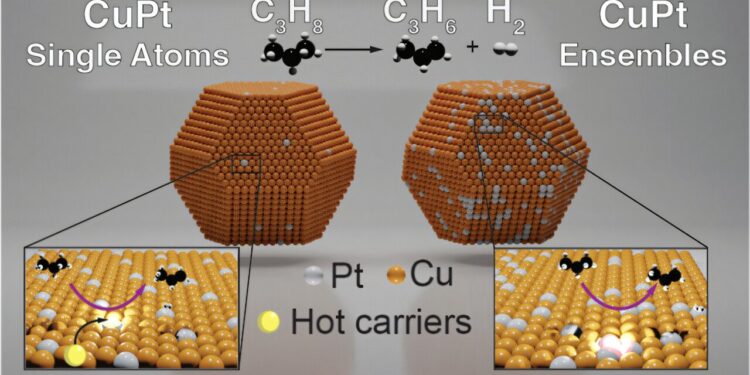
Graphic summary. Credit: Journal of the American Chemical Society (2025). DOI: 10.1021 / jacs. 4C14842
More than 150 million metric tonnes of propylene are produced each year, making it one of the most widespread chemicals used in the chemical industry.
Propylene is the basis of polypropylene, a polymer used in everything, from medical devices to packaging through household items. But most propylene is produced by a steam cracking, a high energy process that uses heat to decompose crude oil with smaller hydrocarbons.
Now, chemists from the Northwest University have found a way to create propylene using light. Their results show that a nanogenerated photoactive catalyst can make propylene directly by a process called non -oxidizing propane dehydrogen (PDH).
The team found that it could catalyze the reaction to create propylene and hydrogen from propane with a chemical process based on light. If it is adopted on a large scale, the process could lead to a drop in emissions from the chemical industry, an important step towards industrial decarbonization.
The results are published today in the Journal of the American Chemical Society. The first authors understand the graduate student Emma-Rose Newmeyer and the postdoctoral researcher Yicheng Wang.
“The propylene is a giant of the chemical industry, and now we show that we can make more soft reactions than those generally used,” said the Sweateur Dayne of Northwestern, who led the study. “This proves that we can take advantage of the designer nanoparticles in our progress towards a more sustainable future. If the industry could use a renewable energy source like light in these catalytic processes, this could further reduce global energy demands.”
Make propylene by channeling light on simple atoms
To make propylene, scientists must decompose carbon-hydrogen bonds at the molecular level. “It does not seem too hard because there are carbon hydrogen links everywhere, but these are very stable links that are difficult to break,” said sweater.
Due to the increase in natural gas availability in the United States thanks to shale resources, the idea of PDH as propylene manufacturing process has become more popular. Not only could this be potentially cheaper, but it could also reduce the need for crude oil resources and help the transition to renewable energies.
However, scientists have struggled to find the right catalysts for this process which would reduce the environmental impact.
For their study, Sweater and his team tested an unexplored idea: to conduct the reaction with a special type of nanoparticles that absorbs light but also has well -defined places where a single atom catalyzes the reaction. The team created an alloy of copper and platinum – a combination known for being a good thermal catalyst – and tested what happened when it shone with light.
They found that when activated with a laser, nanoparticles had become excited and catalyzed the reaction to create propylene.
The team experienced different quantities of allied platinum, as well as the color and intensity of light. They found that when they included isolated platinum atoms in copper nanoparticles, the structure channeled light to isolated platinum atoms, allowing carbon hydrogen bond to break more easily.
“It is this funnel of light energy on unique atoms that allows this reaction to occur,” said sweaty.
And although they tested systems with more platinum, the structures that only included unique platinum atoms worked best – which means this process would only need a small amount of precious metal “without sacrificing reactivity and selectivity,” said Newmeyer.
An additional bonus: the process also creates hydrogen at the same time, offering a secondary secondary by-product.
Potential energy saving for industry
The team also found that they could reduce the overall temperature by 50 degrees Celsius (standard operating temperatures) and obtain the same conversion rate. This means that if this process was adopted by the industry, it could lead to major energy savings.
“It has the potential to have a strong impact on emissions associated with the manufacture of chemicals by reducing the temperatures to which these industrial levels work,” said Newmeyer.
Then, the team hopes to continue to develop this catalyst and test them with other processes that are important to manufacture the constituent elements of the chemical industry.
“There is a lot of room to explore the use of these unique atom alloys focused on light to generate different reactions,” said sweater.
More information:
Emma -Rose Newmeyer et al, location of plasmonic loads and activation C – H at unique atom sites in diluted copper plate alloys, Journal of the American Chemical Society (2025). DOI: 10.1021 / jacs. 4C14842
Supplied by the Northwestern University
Quote: Illuminating unique atoms for the production of sustainable propylene (2025, April 1) recovered on April 2, 2025 from
This document is subject to copyright. In addition to any fair program for private or research purposes, no part can be reproduced without written authorization. The content is provided only for information purposes.