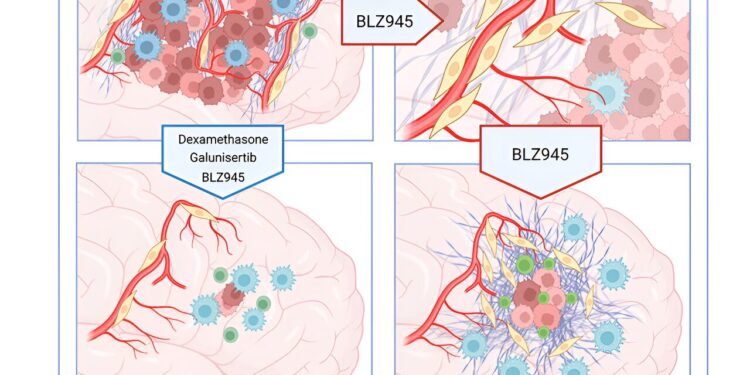
Graphic summary. Credit: Cancer cell (2024). DOI: 10.1016/j.ccell.2024.08.012
A study led by Ludwig Cancer Research has found that recurrent tumors of glioblastoma multiforme (GBM), an aggressive brain cancer, develop from the fibrous scars of malignant predecessors destroyed by interventions such as radiation, surgery and immunotherapy.
Edited by Johanna Joyce, Spencer Watson and Ludwig Lausanne alumnus Anoek Zomer and published in the current issue of Cancer cell—where it appears on the cover—the study describes how these scars enable tumor regrowth and identifies drug targets to sabotage their malignant support. It also demonstrates the efficacy of such combination therapies in preclinical trials using mouse models of GBM.
“We have identified fibrous scarring as a key source of GBM resurgence after treatment, showing how it creates a protective niche for tumor regrowth,” Joyce said. “Our results suggest that blocking the scarring process in the brain by adding anti-fibrosis agents to current treatment strategies could help prevent glioblastoma recurrence and improve treatment outcomes.”
There is an urgent need for such interventions. GBM is the most common and aggressive form of brain cancer in adults. Despite considerable efforts to develop effective therapies for this cancer, the average life expectancy of patients remains approximately 14 months after diagnosis.
The origins of the current study date back to 2016, when the Joyce lab reported in the journal Science Investigating strategies to overcome resistance to a promising immunotherapy for the treatment of GBM in mouse models. This experimental therapy, which inhibits colony-stimulating factor 1 receptor (CSF-1R) signaling and is currently being evaluated in clinical trials, targets immune cells called macrophages and their brain-resident counterparts, microglial cells, both of which are manipulated by GBM cells to support tumor growth and survival.
The Joyce lab has demonstrated that inhibition of CSF-1R reprograms these immune cells into an anti-tumor state and thereby induces significant tumor regression. Science The study found that about half of the mice relapsed after an initial response to therapy. “What was most remarkable about this observation was that every time a brain tumor came back after immunotherapy, it grew back right next to a scar that had formed at the original tumor site,” Joyce said.
In the current study, Joyce, Watson, Zomer, and colleagues examined tumor samples obtained from patients undergoing therapy for GBM and showed that fibrous scars also occur after therapy in humans and are also associated with tumor recurrence. They also showed that fibrous scars occur not only in response to immunotherapy, but also after surgical and radiological removal of tumors.
To study how fibrosis contributes to relapse, the researchers applied an integrated suite of advanced technologies to analyze the cellular and molecular geography of scars and the microenvironment of resurgent tumors.
These technologies include analysis of global gene expression in single cells, comprehensive protein analysis in tissues, and an AI-based workflow and suite of analytical methods for spatial tissue analysis called hyperplexed immunofluorescence imaging (HIFI). Recently developed by Watson and colleagues in the Joyce lab, HIFI enables the simultaneous visualization of multiple molecular markers in and around cells across large tissue cross-sections, enabling the generation of granular maps of the tumor microenvironment.
“Taken together, these advanced methods allowed us to see exactly how fibrous scars form,” Watson said. “They revealed that fibrosis serves as a sort of protective cocoon for residual cancer cells, pushing them into a dormant state in which they are largely resistant to treatment. We found that it also protects them from surveillance and elimination by the immune system.”
Integrated analyses of the tissue microenvironment after therapy revealed that descendants of cells associated with blood vessels feeding tumors become functionally altered to resemble fibroblasts, fiber-producing cells typically involved in wound healing.
These perivascular-derived fibroblast-like cells (PDFL) deploy into the region previously occupied by the regressing tumor, where they promote the formation of fibrous scars. The researchers found that these cells are particularly activated by neuroinflammation and immune factors known as cytokines, including transforming growth factor-β (TGF-β).
“To see if targeting fibrotic scars could improve GBM treatment outcomes, we designed a treatment regimen using existing drugs to block TGF-β signaling and suppress neuroinflammation in combination with CSF-1R inhibition and evaluated it in preclinical trials using mouse models of GBM,” Joyce said.
“We also timed these additional treatments to coincide with the period of peak PDFL activation identified by our studies. Our results show that the drug combination inhibited fibrotic healing, decreased the number of surviving tumor cells, and prolonged survival of treated mice compared to controls.”
The researchers suggest that approaches aimed at limiting fibrotic scarring could significantly improve outcomes for GBM patients receiving surgical, radiation, or macrophage-targeted treatments. Further research, they note, will likely yield even better drug targets for these combination therapies.
More information:
Spencer S. Watson et al, Fibrotic response to anti-CSF-1R therapy potentiates glioblastoma recurrence, Cancer cell (2024). DOI: 10.1016/j.ccell.2024.08.012
Provided by Ludwig Cancer Research
Quote:How Scars from Destroyed Brain Tumors Cause Relapses (2024, September 9) retrieved September 9, 2024 from
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without written permission. The content is provided for informational purposes only.