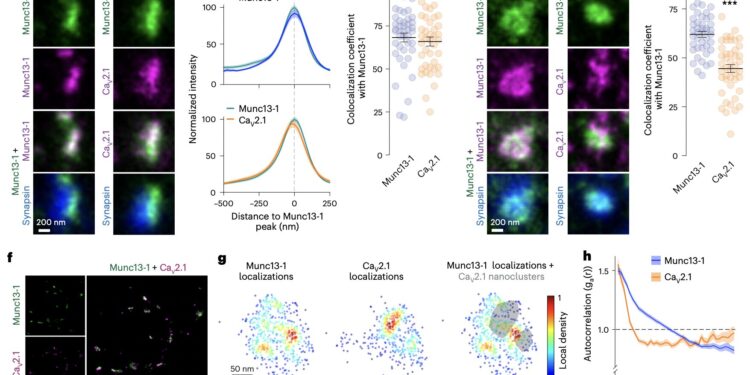
Nanoclustering of Munc13-1 and CaV2.1. a–c, Example STED images of synapses in lateral view (a), quantification of line profiles (b), and Pearson colocalization coefficients (c). Cultured hippocampal neurons stained with antibodies against synapsin and Munc13-1, and a second Munc13-1 antibody or a CaV2.1 antibody. Synapses in lateral view were identified by the presence of an elongated Munc13-1 object (shared Munc13-1 antibody) at the edge of the synapsin-positive vesicle cloud. A line profile (750 nm × 250 nm) was positioned perpendicular to the center of Munc13-1, and signals were aligned to the Munc13-1 peak in the averaged line profiles (b); Munc13-1, 42 synapses/3 cultures; CaV2.1, 42/3. d, e, Same as a–c, but for en face synapses identified by the presence of a Munc13-1 (shared Munc13-1 antibody) area surrounded by synapsin and without line profiles. Munc13-1, 43/3; CaV2.1, 44/3. f, Zoomed-out images rendered by DNA Exchange-PAINT after staining for Munc13-1 and CaV2.1. g, Enlarged view of the density of protein localizations in an example of an en face synapse imaged with DNA ExchangePAINT showing Munc13-1, CaV2.1, and Munc13-1 with shaded CaV2.1 nanoaggregates identified with a density-based clustering algorithm. h, Autocorrelation of synaptic densities of Munc13-1 and CaV2.1 measured as the average probability of detecting localizations for the same protein at increasing distances from any given localization. Munc13-1, 147 synapses/3 cultures; CaV2.1, 147/3. Credit: Emperador-Melero et al. (Neuroscience of Nature(Springer, 2024).
In neuroscience, the term “active zone” refers to a specialized area of the presynaptic membrane of synapses (i.e., the connections through which neurons transmit electrical nerve signals to each other or to other cells). The active zone is the site where neurotransmitters are released at synapses, so it plays a central role in communication between neurons.
During an action potential (i.e., an electrical signal that travels across the neuron’s membrane to transmit information throughout the nervous system), synaptic vesicles fuse with the presynaptic membrane in response to an influx of Ca2+ ions. This process, which takes place in the active zone, ultimately leads to the release of neurotransmitters into the small space between the neuron sending an electrical signal and the neuron receiving it, known as the synaptic cleft.
Researchers from Harvard Medical School and the University of Maryland School of Medicine recently conducted a study of the protein mechanism in the active zone that mediates synaptic vesicle priming and Ca clustering.2+ channels. Their findings, published in Neuroscience of Natureunveil two distinct active zone protein machines at hippocampal synapses that independently perform these functions.
“Action potentials trigger neurotransmitter release in the presynaptic active zone with spatiotemporal precision,” Emperador-Melero, Jonathan W. Andersen and colleagues wrote in their paper.
“This is supported by the protein machinery that ensures synaptic vesicle priming and Ca clusteringV2 Ca2+ nearby channels. One model postulates that scaffold proteins directly tether the vesicles to CaV2s ; however, we find here that at mouse hippocampal synapses, CaV“Vesicle clustering and priming are performed by separate machines.”
In their studies, the researchers examined floxed mice and pre-prepared hippocampal cultures extracted from mouse pups using various microscopy, imaging and processing techniques. Their analyses showed that two proteins, namely Cav2 and Munc13 each form nanoclusters positioned at varying distances from each other at hippocampal synapses.
“CaliforniaV“2 nanoclusters are positioned at varying distances from those of the priming protein Munc13,” Emperador-Melero, Andersen, and colleagues wrote.
“The active zone organizer RIM anchors both proteins but distinct interaction motifs independently execute these functions. In transfected cells, liprin-α and RIM form separate co-assemblies of the CaV2-organizer complexes. At synapses, inactivation of liporin-α1–liprin-α4 impairs vesicle priming but not CaV2 clustering. Cell adhesion protein PTPσ recruits liprin-α, RIM, and Munc13 into priming complexes without Ca clusteringV2s.”
Based on their observations, the researchers conclude that CaVThe 2 clusters and vesicle priming sites are independently organized and are supported by distinct mechanisms. They also concluded that Liprin-α and PTPσ proteins specifically support the assembly of priming sites.
Overall, these recent findings suggest that there are at least two distinct active zone mechanisms, marked by independent mechanisms that mediate Ca2+ channel clustering and vesicle priming. The researchers hypothesize that the active zone substructuring they observed contributes to the resilience of synapse assembly and the active zone reported in various previous neuroscience studies.
The results collected in this research effort could pave the way for further studies to investigate in more detail the different protein machines identified in the active zone. This could lead to more interesting observations on the substructuring of the active zone, and potentially to the identification of other protein machines mediating Ca2+ channel clustering and vesicle priming.
More information:
Javier Emperador-Melero et al, Distinct active zone protein machineries are involved in Ca2+ channel clustering and vesicle priming at hippocampal synapses, Neuroscience of Nature (2024). DOI: 10.1038/s41593-024-01720-5
© 2024 Science X Network
Quote:Hippocampal study unveils distinct protein mechanisms for calcium channel clustering and vesicle priming (2024, September 11) retrieved September 11, 2024 from
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without written permission. The content is provided for informational purposes only.