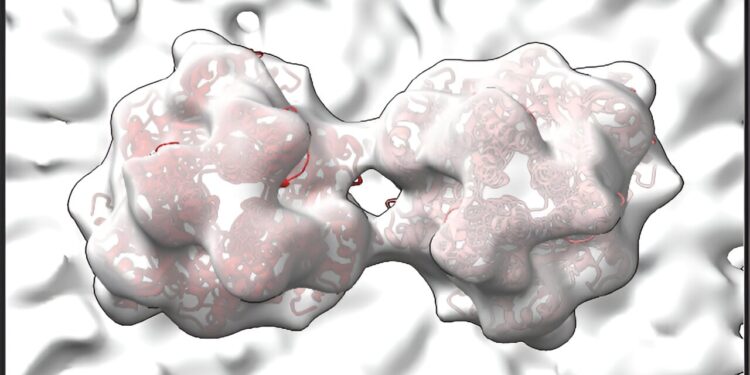
RSV’s F proteins, shown in this image created by University of Wisconsin-Madison researchers using a technique called cryo-electron tomography, may make RSV more potent by preventing it from prematurely infecting cells. Credit: Wright Lab, UW–Madison
The complex form of respiratory syncytial virus (RSV) poses a barrier limiting the development of treatments for an infection that results in hospitalization or worse for hundreds of thousands of people in the United States each year, according to the Centers for Disease Control and Prevention. New images of the virus taken by researchers at the University of Wisconsin-Madison could be key to preventing or slowing RSV infections.
RSV is of particular concern in young children, the elderly, and adults at high risk of respiratory complications. Yet unlike the flu and other common communicable respiratory illnesses that plague schools every year, there are few options to combat RSV. In the United States, prophylactic treatments are available for young children and existing vaccines are only approved for pregnant women and the elderly.
The structure of the virus, made up of tiny curved filaments, has eluded researchers. This has made it difficult to identify key drug targets, including viral components conserved in related viruses.
“There are a number of viruses related to RSV that are also important human pathogens, including measles,” says Elizabeth Wright, a professor of biochemistry at UW-Madison. “What we know about related viruses gives us clues about RSV protein structures, but to identify drug targets, we need to look more closely at RSV proteins that are intimately associated with host cell membranes.”
Using an imaging technique called cryo-electron tomography, Wright and his team revealed details about the molecules and structures essential to the form and function of RSV. They published their findings in Natural communication.
Mean RSV M subtomogram. Credit: Natural communications (2024). DOI: 10.1038/s41467-024-50162-x
Cryo-ET freezes virus particles or other molecules at ultra-cold temperatures, stopping biological processes in action. This allows researchers to examine the structures of organisms, cells and organelles, as well as viruses, and capture small-scale images of structures frozen in time. Instantly freeze many RSV particles and cryo-ET imaging will capture (almost) every possible configuration of the virus from many different angles. These 2D images are combined to produce a high-resolution representation of the 3D structures of the virus, even down to the level of individual atoms.
Wright’s recent study produced high-resolution images detailing the structure of two RSV proteins, the RSV M protein and the RSV F protein, which are essential for the interaction between the virus and the host cell membrane. Both proteins are also present in related viruses.
The RSV M protein interacts with host cell membranes, holding the filamentous structure of the virus together and coordinating viral components and other proteins, including the RSV F proteins. The RSV F proteins are found on the surface of the virus, ready to interact with host cell receptors and regulate virus fusion and entry into the host cell. The scientists’ images reveal that in RSV, two F proteins come together to form a more stable unit. Wright says this association could prevent F proteins from prematurely infecting the host cell.
“Our main findings reveal structural details that allow us to better understand not only how the protein regulates the assembly of viral particles, but also the coordination of proteins that allow the virus to be infectious,” says Wright.
Scientists believe that F protein pairs could be the key to destabilizing the virus before it is ready to infect its next host, making F protein pairs a possible target for future drug development. They will continue to explore how RSV proteins interact with each other to cause infection.
More information:
Bryan S. Sibert et al, Assembly of the respiratory syncytial virus matrix protein network and its coordination with fusion glycoprotein trimers, Natural communications (2024). DOI: 10.1038/s41467-024-50162-x
Provided by University of Wisconsin-Madison
Quote: High-resolution images of RSV may reveal weak spots of stubborn virus (October 1, 2024) retrieved October 1, 2024 from
This document is subject to copyright. Except for fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for informational purposes only.