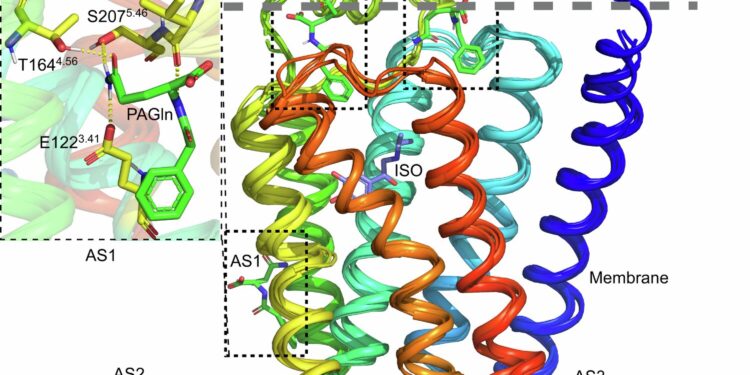
Docking of PAGln to extracellular candidate binding sites and known allosteric sites of β2AR. Credit: Nature Communications (2024). DOI: 10.1038/s41467-024-50855-3
Researchers at the Cleveland Clinic have made an important discovery about how the gut microbiome interacts with cells to cause cardiovascular disease. The study, published in Nature Communications They discovered that phenylacetylglutamine (PAG), produced by gut bacteria as a waste product and then absorbed and formed in the liver, interacts with previously unknown locations on beta-2 adrenergic receptors on heart cells once it enters the circulation.
PAG has been shown to interact with beta-2 adrenergic receptors to influence the force of contraction of heart muscle cells, a process that researchers believe contributes to heart failure. The researchers showed that mutating parts of the beta-2 adrenergic receptor, previously thought to be unrelated to signaling activity in preclinical models, prevented PAG from depressing the receptor’s function.
This is the latest in a series of PAG studies led by Dr. Stanley Hazen, chair of cardiovascular and metabolic sciences at the Cleveland Clinic’s Lerner Research Institute and co-director of the Section of Preventive Cardiology. Dr. Hazen’s lab has previously shown that high circulating levels of PAG in subjects are associated with an increased risk of developing heart failure and lead to worse outcomes for patients with heart failure.
They also showed that the gut microbial PAG signaling pathway was mechanistically linked to many features associated with heart failure and cardiovascular disease risk. These new findings bring us one step closer to therapeutically targeting this pathway to develop better treatment for heart failure prevention, says Dr. Hazen.
Beta-2 adrenergic receptor blockade is imperfect
Beta-blockers, a drug commonly used to treat heart failure and high blood pressure, target our body’s “fight or flight” response. This essential response is controlled by beta-adrenergic receptors and is fundamental to survival, but repeated instances of fight or flight over time can lead to chronic damage to the heart, contributing to the development of heart failure. Dr. Sathyamangla Prasad, who contributed to the study, brought his expertise on beta-adrenergic receptors and heart failure to the investigation.
A standard treatment to relieve stress on the heart is a beta-blocker drug, which works like an on/off switch for beta-2 adrenergic receptors. To activate the fight-or-flight pathway, hormones like epinephrine bind directly to specially designed slots in beta-2 adrenergic receptors, like a key sliding into a lock.
Beta-blockers are designed to fit into the same keyhole, blocking adrenaline and other hormones from binding to beta-2 adrenergic receptors. This in turn slows the heart rate, reduces strain on the heart, and opens blood vessels. Previous studies by this research team found that circulating PAG levels were associated with the presence of heart failure and severity indices, and that PAG directly promoted heart failure-related features, including a weakened heart rate. The adverse effects of PAG on heart failure-related features were reversed using a common beta-blocker in preclinical models, reinforcing the link between PAG, heart failure, and beta-adrenergic receptors.
New PAG Discovery Offers Additional Potential Therapeutic Options
Current studies are looking to further determine how PAG interacts with our beta-adrenergic receptors. Prasenjit Saha, Ph.D., first author and a member of Dr. Hazen’s lab, mutated different areas of the beta-2 adrenergic receptor and tested whether signaling could occur with the natural hormone epinephrine (also known as adrenaline). Preclinical testing showed that mutating certain locations kept the adrenaline binding site both intact and functional, but the mutant receptor was no longer downregulated by PAG.
According to Dr. Hazen, these results indicate that beta-2 adrenergic receptors may be regulated by a second PAG binding site that acts as a customized “dimmer switch” for the adrenaline signaling pathway. Because PAG interacts with the receptor at a different location than the main hormone, adrenaline, Dr. Hazen hypothesizes that they could be independently targeted to block PAG signaling generated by harmful gut microbes while allowing the body’s natural adrenaline signals to pass through.
Dr. Hazen says his team’s findings open the door to a whole new way of developing drugs that regulate the beta-2 adrenergic receptor, a more nuanced way than what’s currently on the market. They’re now working on developing drugs that target the PAG pathway and its interactions with adrenergic receptors as a new form of treatment for cardiovascular disease.
“A more targeted beta blocker to block harmful adrenergic receptor signaling, while allowing healthy signals to pass through, would be an entirely new approach to treating or preventing cardiovascular disease risk,” says Dr. Hazen. “This could improve the quality of life for patients who rely on beta blockers to calm their body’s stress responses.”
More information:
Prasenjit Prasad Saha et al, Gut microbe-generated phenylacetylglutamine is an endogenous allosteric modulator of β2-adrenergic receptors, Nature Communications (2024). DOI: 10.1038/s41467-024-50855-3
Provided by Cleveland Clinic
Quote:Gut microbial pathway identified as target for better treatment of heart disease (2024, August 20) retrieved August 20, 2024 from
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without written permission. The content is provided for informational purposes only.