A surge of glucose activity precedes mouse gastrulation. Credit: Nature (2024). DOI: 10.1038/s41586-024-08044-1
Yale University researchers have discovered that glucose metabolism plays a critical role in the early development of mouse embryos, revealing that specific metabolic pathways regulate essential cell signaling during key phases of embryogenesis.
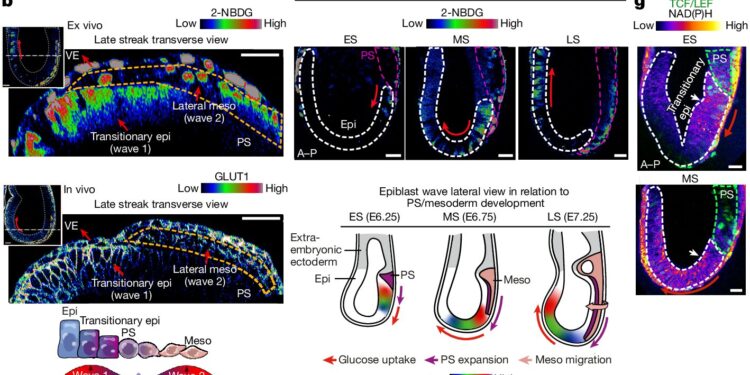
The study, “Selective use of glucose metabolism guides mammalian gastrulation,” published in Natureidentifies two distinct waves of glucose utilization during mouse gastrulation.
The first wave channels glucose through the hexosamine biosynthesis pathway (HBP) in epiblast cells, thereby facilitating the formation of proteoglycans for fibroblast growth factor (FGF) signaling. This signaling is crucial for cell differentiation and extension of the primitive streak. This structure ultimately forms the neural plate, which becomes the basis of the spinal cord and nervous system.
The second wave directs glucose to glycolysis, providing the energy and metabolites necessary for mesodermal cell migration and lateral expansion.
The team mapped the spatial and temporal patterns of glucose uptake using single-cell resolution imaging of developing mouse embryos, stem cell models, and embryo-derived tissues.
They found that the initial wave of glucose metabolism occurs in posterior epiblast cells and builds as development progresses. The subsequent wave of glycolysis promotes the movement of mesodermal cells away from the primitive streak, thereby promoting lateral expansion.
To test the certainty of the pathway’s implications, the team inhibited glucose metabolism using chemical blockers, experimentally confirming disruption of primitive streak formation and mesoderm specification.
Specific targeting of HBP impaired primitive streak development while inhibiting late-stage glycolysis with affected mesodermal cell migration without hindering initial cell fate decisions.
The study also demonstrates that glucose metabolism influences extracellular signal-regulated kinase (ERK) signaling pathways, finding them crucial for cell differentiation and movement during gastrulation. Inhibition of glucose metabolism resulted in reduced ERK activity, while supplementation with N-acetylglucosamine, a product of HBP, restored ERK signaling and rescued developmental defects.
Further analysis using stem cell-based embryo models and mesoderm explants confirmed that HBP is essential for epiblast cell fate transitions and that glycolysis supports the migratory behavior of mesodermal cells.
RNA sequencing of treated mesoderm explants revealed downregulation of pathways involved in cell migration and extracellular matrix interactions when glycolysis or ERK signaling was inhibited.
The results highlight that glucose metabolism, in coordination with genetic and signaling mechanisms, is integral to the successful patterning and morphogenesis of the developing embryo. This research challenges the traditional view of cellular metabolism as simply a background cellular function, positioning it instead as an active director of embryonic development.
More information:
Dominique Cao et al, The selective use of glucose metabolism guides mammalian gastrulation, Nature (2024). DOI: 10.1038/s41586-024-08044-1
Christian Schröter, Glucose plays a surprising role in directing cell fate and migration, Nature (2024). DOI: 10.1038/d41586-024-03284-7
© 2024 Science X Network
Quote: Glucose metabolism stimulates embryonic development in mice, reveals study (October 19, 2024) retrieved October 19, 2024 from
This document is subject to copyright. Except for fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for informational purposes only.