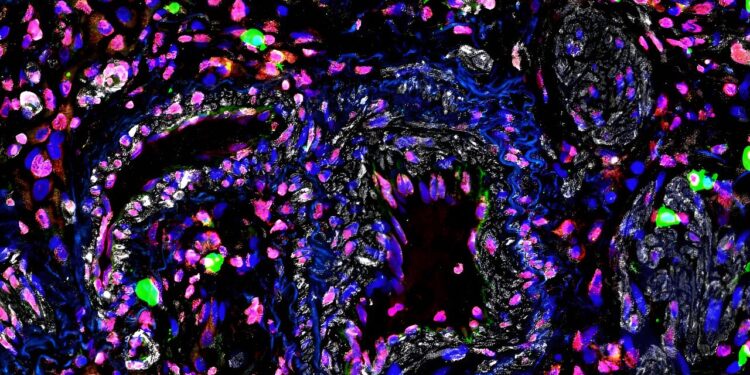
Immunofluorescent staining of the ncRNA fragment in a diseased pulmonary vessel from a patient with pulmonary arterial hypertension. Credit: Thomas Bertero
Researchers at the University of Pittsburgh Schools of Medicine have discovered a fundamental mechanism that controls the body’s response to limited oxygen and regulates disease in the blood vessels of the lungs.
By combing the genomes of more than 20,000 individuals in the United States, France, England and Japan and combining the results with molecular laboratory studies, the team discovered a common genetic trait that could predict a higher risk of a disease of the small vessels of the lungs called pulmonary hypertension. and its more serious form, pulmonary arterial hypertension, and influence the development of drug therapies targeting the body’s response to a lack of oxygen. The results were published in Scientific translational medicine.
“This new level of knowledge will help identify individuals who may be at higher genetic risk for pulmonary hypertension and jump-start precision medicine practices to deliver personalized treatments,” said lead author Stephen Chan, MD, Ph.D., a cardiologist who holds the Vitalant Chair in Vascular Medicine and is director of Pitt’s Vascular Medicine Institute.
Pulmonary hypertension encompasses a range of conditions of various causes that manifest as high blood pressure in the arteries of the lung and the right side of the heart. The disease is accompanied by a decrease in oxygen supply to lung tissues and blood, is chronic and fatal, and its molecular origins and genetic background remain unresolved.
Chan Laboratory at the University of Pittsburgh. Credit: Johnathan Wright
Using a combined approach of genomics and biochemistry, the Chan lab discovered a pair of genes that played an important role in regulating blood vessel metabolism and disease. This pair of genes included a long noncoding RNA molecule—a messenger that facilitates the transformation of the body’s genetic code into protein products—and a protein-binding partner, and their interaction was frequently active in cells exposed to low oxygen content compared to normal cells.
Taking the results a step further, the team found that a single DNA letter change directing the expression of this RNA-protein pair under low-oxygen conditions was associated with higher genetic risk. of pulmonary hypertension in various patient populations.
According to Chan, pulmonary hypertension is an orphan disease, and the limited number of patients with pulmonary hypertension makes it difficult to find genetic variations that are rare but still large enough to overshadow individual differences.
With this in mind, Pitt scientists turned to collaborators around the world and to public research datasets such as All of Us, a national health registry, to ensure the results are relevant to a diverse global population .
Chan hopes that his findings will spur the development of targeted therapies relevant to oxygen sensitivity in the lining of blood vessels and that their pending patent application will contribute to the growth of an entirely new field of epigenetic and RNA drug therapies which work not by manipulating the genome but by changing the way it is read.
More information:
Yi Yin Tai et al, Allele-specific control of rodent and human lncRNA KMT2E-AS1 promotes hypoxic endothelial pathology in pulmonary hypertension, Scientific translational medicine (2024). DOI: 10.1126/scitranslmed.add2029. www.science.org/doi/10.1126/scitranslmed.add2029
Provided by the University of Pittsburgh
Quote: Genetics may influence the body’s response to a lack of oxygen, study finds (January 10, 2024) retrieved January 10, 2024 from
This document is subject to copyright. Apart from fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.