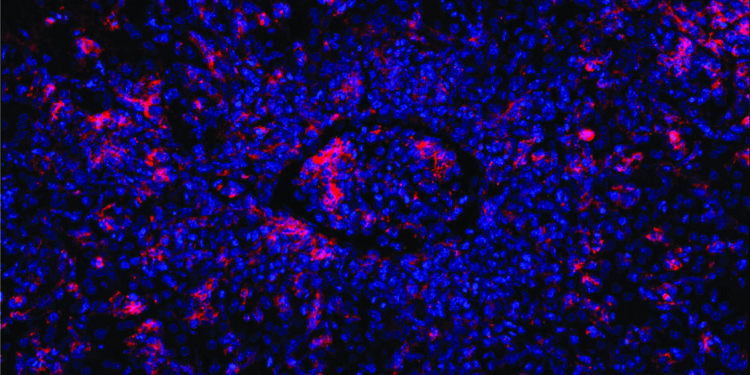
Mutated T cells are unable to kill B cells (red) induced by the Epstein-Barr virus. This causes other immune cells to rush into the infected area, blocking a blood vessel (center). Credit: Elijah D. Lowenstein and Xun Li, K. Rajewsky Lab, Max Delbrück Center
Some inherited genetic abnormalities cause an exaggerated immune response that can be fatal. Thanks to the gene editing tool CRISPR-Cas9, these defects can be corrected, thereby normalizing the immune response, as researchers led by Klaus Rajewsky of the Max Delbrück Center now report in Scientific immunology.
Familial hemophagocytic lymphohistiocytosis (FHL) is a rare disease of the immune system that usually occurs in infants and young children younger than 18 months. The disease is serious and carries a high mortality rate. It is caused by various genetic mutations that prevent cytotoxic T cells from functioning normally. These are a group of immune cells that kill virus-infected cells or otherwise damaged cells.
If a child with FHL contracts a virus, such as Epstein-Barr virus (EBV), but also other viruses, the cytotoxic T cells cannot eliminate the infected cells. Instead, the immune response goes out of control. This leads to a cytokine storm and excessive inflammatory response that affects the entire body.
“Doctors treat FHL with a combination of chemotherapy, immunosuppression and bone marrow transplantation, but many children still die from the disease,” explains Professor Klaus Rajewsky, who heads the Immune Regulation and Cancer Laboratory at the Max Delbrück Center.
He and his team therefore developed a new therapeutic strategy. Using the gene-editing tool CRISPR-Cas9, researchers successfully repaired defective T cells from mice and two critically ill infants. The repaired cytotoxic T cells then functioned normally, with the mice recovering from hemophagocytic lymphohistiocytosis.
Gene repair strategy works in mice
The starting point for the study was mice in which the team could mimic EBV infections. In these animals, researchers modified a gene called perforin so that its function was completely lost or severely compromised, a common genetic abnormality in patients with FHL.
When they then caused a condition resembling an EBV infection, the affected B cells multiplied out of control because the defective cytotoxic T cells were unable to eliminate them. As a result, the immune response was accelerated and the mice developed hemophagocytic lymphohistiocytosis.
The team then collected memory T stem cells, that is, long-lived T cells from which active cytotoxic T cells can mature, from the mice’s blood. The researchers used the gene-editing tool CRISPR-Cas9 to repair the defective perforin gene in memory T cells, then reinjected the corrected cells into mice. The animals’ immune response calmed and their symptoms disappeared.
The duration of protection is uncertain
The paper’s first author, Dr. Xun Li, used blood samples from two sick infants to test whether the strategy also worked in humans. One had a defective perforin gene, the other a different defective gene.
“Our gene repair technique is more precise than previous methods and T cells remain virtually unchanged after undergoing genetic modification,” explains Li. “It was also fascinating to see how efficiently memory T cells could be expanded and repaired, even from a small amount of blood.”
Cell culture experiments showed that repaired memory T cells from infants were capable of a normal cytotoxic T cell response.
This means that the therapeutic mechanism works in principle. But before patients can benefit from this discovery, the team must first resolve open questions and test the treatment concept in clinical trials.
“It is still unclear exactly how long the protective effect lasts,” says Dr. Christine Kocks, a scientist on Rajewsky’s team. “Since memory T stem cells remain in the body for a long time, we hope that the therapy will provide long-term or even permanent protection. It is also conceivable that patients could be treated again and again with their repaired T cells. “
The procedure is minimally invasive, as only a small amount of blood is needed and the mice did not require any preparatory treatment, unlike, for example, a bone marrow transplant.
“We sincerely hope that our mechanism of action will be a major breakthrough in the treatment of FHL,” says Rajewsky, “either to buy more time for a successful bone marrow transplant or even as a treatment itself.”
More information:
Xun Li et al, Precise CRISPR-Cas9 gene repair in autologous memory T cells to treat familial hemophagocytic lymphohistiocytosis, Scientific immunology (2024). DOI: 10.1126/sciimmunol.adi0042. www.science.org/doi/10.1126/sciimmunol.adi0042
Provided by the Max Delbrück Center for Molecular Medicine
Quote: Gene editing precisely repairs immune cells (February 2, 2024) retrieved February 2, 2024 from
This document is subject to copyright. Except for fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.