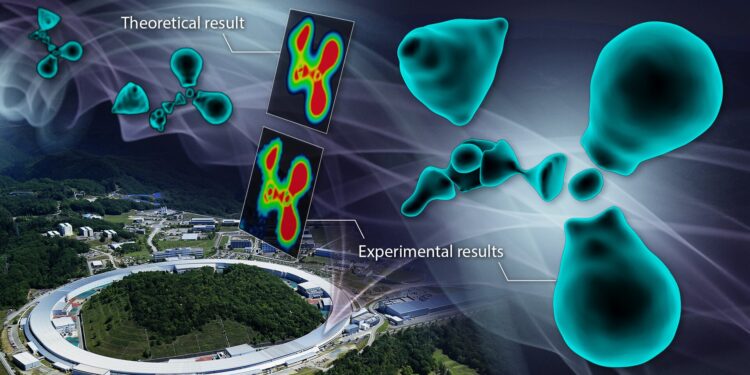
The distribution of outer-shell electrons, called valence electrons, in organic molecules has been observed for the first time. Credit: Reiko Matsushita / RIKEN
The distribution of outer-shell electrons, called valence electrons, in organic molecules has been experimentally observed for the first time by a team led by Nagoya University in Japan. Since interactions between atoms are governed by valence electrons, their findings shed light on the fundamental nature of chemical bonding, with implications for pharmacy and chemical engineering. The results were published in the journal Journal of the American Chemical Society.
The behavior of electrons in atoms is complex, forming electron orbitals that have different functions depending on their proximity to the nucleus. The inner-shell electrons, called core electrons, serve for self-stabilization and do not interact with other atoms. In contrast, the outer electrons, or valence electrons, define most of the material’s properties, especially when bonding with other atoms.
To understand the properties of a material, information about its valence electrons must be extracted. However, it is difficult to experimentally isolate information about valence electrons alone, forcing researchers to rely on theoretical models and spectroscopy to estimate it.
By conducting world-class synchrotron X-ray diffraction experiments at SPring-8, the group discovered that it is possible to selectively extract only the valence electron density of atoms in a crystal.
“We called this method the CDFS method. With it, we observed the electronic state of the glycine molecule, a type of amino acid,” said corresponding author Hiroshi Sawa. “Although the method is relatively simple to implement, the result was impressive. The observed electron cloud did not have the smooth, enveloping shape that many predicted, but rather a fragmented, discrete state.”
Direct observation of the valence electron density was performed by synchrotron X-ray diffraction at SPring-8. Theoretical results obtained using a quantum mechanical modeling method called density functional theory (DFT) were matched with the observation. Credit: Sawa Lab, Nagoya University/RIKEN
To understand the nature of the results, the group created a color map of their observations. In chemistry, a color map uses colors to show how data sets vary over a specific range. These maps are often used in conjunction with spectroscopic, imaging, and chemical analysis techniques to provide an intuitive way to interpret complex data sets.
The cross-sectional view map in the enlarged diagram clearly shows interruptions in the distribution of electrons surrounding the carbon atoms.
“When carbon forms bonds with surrounding atoms, it rebuilds its electron cloud to create hybrid orbitals. In this case, the outermost L-shell electrons have nodes based on their wave nature, known as wave functions,” Sawa explains. “This means that because of the wave nature of electrons, there are parts of the hybrid orbitals where electrons are absent, which is very different from the image many people have of a continuous ‘cloud’ of electrons.”
The fragmented distribution of the electron cloud observed in the experiment demonstrates the wave nature of electron quantum mechanics, as predicted by physics. To confirm whether the observed electron cloud accurately captures the real state, they performed advanced quantum chemistry calculations in collaboration with Hokkaido University that confirmed that the experimental and theoretical results matched perfectly.
Sawa believes the results demonstrate the benefits of interdisciplinary research. “I think this has been helpful in bringing a clear conclusion to the ambiguous understanding of binding states that has puzzled researchers since the 19th century,” Sawa said.
“Visualizing the behavior of electrons is a difficult task, but the results can be elegantly interpreted as electrons acting according to wave functions. I think our findings have surprised many researchers and validated the model proposed by quantum chemistry.”
With a precise understanding of the distribution of the valence electron density forming this molecule, the group conducted similar experiments and calculations on cytidine, a slightly more complex molecule. They managed to extract the electrons within the carbon double bonds and clearly observed the differences between the carbon-carbon and carbon-nitrogen bonds.
“This study has directly visualized the essence of chemical bonds, potentially contributing to the design of functional materials and understanding reaction mechanisms. Indeed, it helps to discuss the electronic states of molecules, which are difficult to deduce from the structural chemical formula alone,” Sawa said.
“This can, for example, explain why some drugs work and others don’t. Areas in which interactions influence functionality and structural stability, such as organic semiconductors and research into the structure of DNA double helices, are likely to benefit most from our research.”
More information:
Hara, T. et al. Unveiling the nature of chemical bonds in real space, Journal of the American Chemical Society (2024). DOI: 10.1021/jacs.4c05673
Provided by Nagoya University
Quote:First Visualization of Valence Electrons Reveals Fundamental Nature of Chemical Bonding (2024, August 21) retrieved August 21, 2024 from
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without written permission. The content is provided for informational purposes only.