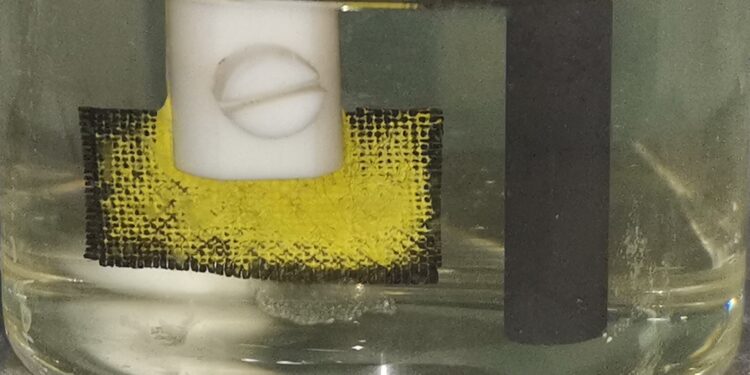
This new coated fabric has actually accumulated uranium (yellow) on its surface from uranium-enriched seawater. Credit: Adapted from ACS Central Science, 2023, DOI: 10.1021/acscentsci.3c01291
The oceans cover most of the Earth’s surface and are home to a staggering number of life forms, but they also harbor a dilute population of uranium ions. And if we can extract these particular ions from water, they could provide a sustainable fuel source for producing nuclear power.
Researchers publishing in ACS Central Science have now developed a material for use with electrochemical extraction that attracts hard-to-obtain uranium ions from seawater more efficiently than existing methods.
Nuclear reactors release the energy naturally stored inside an atom and transform it into heat and electricity by literally breaking the atom apart – a process known as fission. Uranium has become the preferred element in this process because all of its forms are unstable and radioactive, making it easier to split.
Currently, this metal is mined from rocks, but uranium ore deposits are limited. Yet the Nuclear Energy Agency estimates that 4.5 billion tonnes of uranium is floating in our oceans as dissolved uranyl ions. This reserve is more than 1,000 times larger than what is found on earth.
Extracting these ions has proven difficult, however, because the materials used for this do not have enough surface area to effectively trap the ions. So Rui Zhao, Guangshan Zhu and their colleagues wanted to develop an electrode material with many microscopic nooks and crannies that could be used in the electrochemical capture of uranium ions from seawater.
To create their electrodes, the team started with a flexible fabric woven from carbon fibers. They coated the fabric with two specialized monomers which were then polymerized. Next, they treated the fabric with hydroxylamine hydrochloride to add amidoxime groups to the polymers. The natural, porous structure of the fabric created many small pockets in which the amidoxime can nestle and easily trap the uranyl ions.
In experiments, the researchers placed the coated fabric as a cathode in naturally occurring or uranium-enriched seawater, added a graphite anode, and passed a cyclic current between the electrodes. Over time, bright yellow uranium-based precipitates accumulated on the cathode fabric.
In tests using seawater collected from the Bohai Sea, the electrodes extracted 12.6 milligrams of uranium per gram of water for 24 days. The capacity of the coated material was greater than that of most other uranium mining materials tested by the team. Additionally, using electrochemistry to trap the ions was about three times faster than simply letting them accumulate naturally on tissues.
Researchers say the work offers an effective method for capturing uranium from seawater, which could open the oceans to new suppliers of nuclear fuel.
More information:
Self-contained porous aromatic framework electrodes for efficient electrochemical extraction of uranium, ACS Central Science (2023). DOI: 10.1021/acscentsci.3c01291
Provided by the American Chemical Society
Quote: Extracting Uranium from Seawater as an Alternative Nuclear Fuel Source (December 13, 2023) retrieved December 13, 2023 from
This document is subject to copyright. Apart from fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.