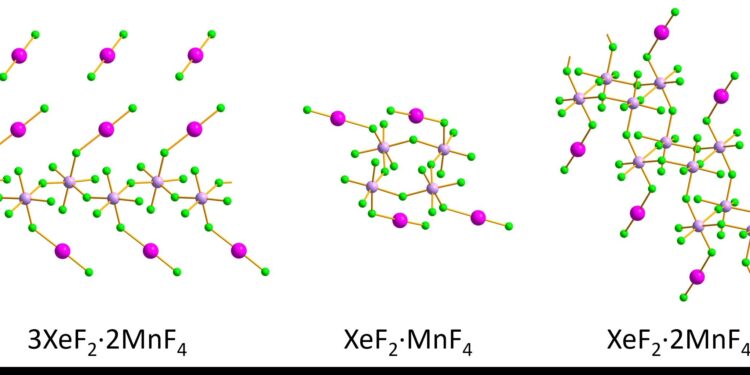
The structures of three xenon compounds have been successfully characterized by 3D electron diffraction. Credit: Matic Lozinšek
Noble gases have a reputation for being inert and unreactive elements, but over 60 years ago, Neil Bartlett demonstrated the first way to bind xenon. He created XePtF6a yellow-orange solid. Because it is difficult to grow sufficiently large crystals containing noble gases, some of their structures – and thus their functions – remain elusive. Now, researchers have successfully examined tiny crystallites of noble gas compounds. They have described the structures of several xenon compounds in Central Scientific Center of the ACS.
Since Bartlett’s discovery, which is commemorated by an international chemical landmark, hundreds of noble gas compounds have been synthesized and some crystal structures have been characterized by single-crystal X-ray diffraction.
However, noble gas crystals are typically sensitive to moisture in the air. This chemical property makes them highly reactive and difficult to manipulate, requiring special techniques and equipment to grow crystals large enough for X-ray diffraction analysis. As a result, the detailed structures of this first xenon compound and several other noble gas compounds have eluded researchers.
Recently, another technique, 3D electron diffraction, revealed the structure of small nanoscale crystals. These crystallites are stable in air, but this technique has not been widely applied to air-sensitive compounds. Lukáš Palatinus, Matic Lozinšek and their colleagues therefore wanted to test 3D electron diffraction on crystallites of xenon-containing compounds.
The researchers synthesized three compounds of xenon difluoride and manganese tetrafluoride, yielding individual red crystals and pink crystalline powders. The samples were kept stable by first cooling a support with liquid nitrogen, adding the sample, and then covering the filled support with several protective layers while transferring it to a transmission electron microscope.
The team measured the bond lengths and angles of xenon fluoride (Xe–F) and manganese fluoride (Mn–F) for nanometer-sized crystallites in the pink crystalline powder using 3D electron diffraction. The structures were then compared to the team’s results on the larger, micrometer-sized wine-red crystals using single-crystal X-ray diffraction. The two methods agreed well, despite small differences, the researchers said, and the results showed that the structures were:
- Infinite zigzag chains for 3XeF2·2 million francs4.
- Rings for XeF2·MnF4.
- Double Stair-Shaped Chains for XeF2·2 million francs4.
Following this successful demonstration of 3D electron diffraction on xenon compounds, the researchers say the technique could be used to uncover the structures of XePtF6 and other difficult-to-characterize noble gas compounds that have eluded characterization for decades, as well as other air-sensitive substances.
More information:
Reactive noble gas compounds explored by 3D electron diffraction: XeF2−MnF4 adducts and simple sample handling procedure, Central Scientific Center of the ACS (2024). DOI: 10.1021/acscentsci.4c00815
Provided by the American Chemical Society
Quote: Exploration of the structures of xenon-containing crystallites (2024, August 14) retrieved August 14, 2024 from
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without written permission. The content is provided for informational purposes only.