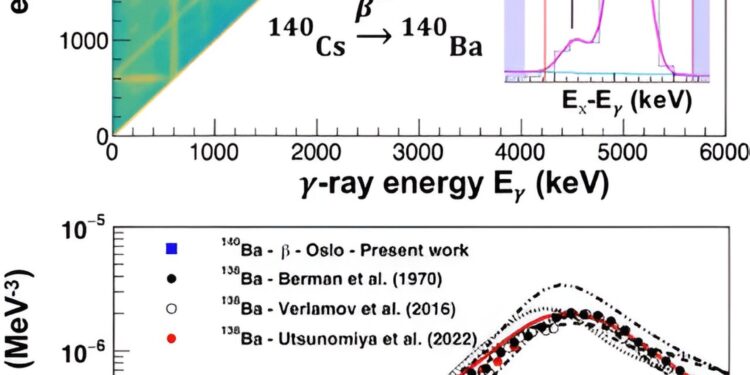
Top: Raw matrix of 𝛾-ray energies and excitation energies after 𝛽-decay of 140Cs. The two diagonal projections for excitation energies from 4.0 to 4.4 MeV are shown as insets with their fits. Bottom: 𝛾SF extracted in the present work (blue squares) compared to data for 138Ba at higher energies (black, white, and red dots), as well as to theoretical models from talys1.95. Credit: Physical Exam Letters (2024). DOI: 10.1103/PhysRevLett.132.202701
How are stars born and die? How do they produce the energy that keeps them alive for billions of years? How do they create the elements we see today? Definitive answers to these questions continue to elude scientists in their quest to understand the processes that shape the chemical makeup of the universe.
Although the exact details of the reaction processes are unclear, understanding where and how elements form, as well as the processes of star formation, is essential to having a complete picture of the history, structure and evolution of the universe.
Recently, an international team, including researchers from the U.S. Department of Energy’s (DOE) Argonne National Laboratory, obtained new experimental data that shed light on how some of the heaviest elements in the universe form in stars. This discovery begins to answer fundamental questions about our origins.
The results are published in the journal Physical Exam Letters.
In particular, the team obtained the first experimental constraints to measure the rate of the process in which neutrons collide and fuse with a nucleus of the isotope barium-139 to form barium-140. Isotopes are members of a family of elements that all have the same number of protons but different numbers of neutrons. The rate at which barium-139 reacts as it transforms into barium-140 has been a dominant source of uncertainty in predictive models used to determine the presence of heavy element isotopes in stars.
Led by Artemis Spyrou, a professor in the Department of Physics and Astronomy at Michigan State University and the Facility for Rare Isotope Beams (FRIB), and Dennis Mücher, a professor in the Institute of Nuclear Physics at the University of Cologne in Germany, the team benefited from the use of CARIBU, a sophisticated radioactive ion source located in the Argonne Tandem Linac Accelerator System (ATLAS), a user facility of DOE’s Office of Nuclear Physics at Argonne.
“It is now clear that the synthesis of elements in stars is more complex than previously thought,” Spyrou said. “Only through this type of measurement will we be able to distinguish the contributions of different astrophysical processes.”
Scientists have long known that the heavy elements in stars, such as barium, lanthanum, and cesium, are created by fast and slow nucleosynthesis processes. Nucleosynthesis is the formation of new atomic nuclei (the centers of atoms composed of protons and neutrons), or elements, by various processes in the universe.
The fast or “r” process, which takes place in seconds, is thought to be responsible for nucleosynthesis in exploding stars, such as supernovae, and small, dense stars that emerge after their collapse. Conversely, the slow or “s” process is thought to be responsible for nucleosynthesis mainly in older, very bright stars near the end of their lives.
Relatively recent astronomical observations suggest a nucleosynthesis pathway that is different from the fast and slow processes. Because some stars considered to be metal-poor exhibit unusual abundance profiles of certain elements, scientists have proposed an intermediate or “i” process to explain this phenomenon.
“What fascinates me most is that we find these different elements on Earth and, often without knowing it, interact with them almost daily,” said Mücher. “However, we do not yet fully understand where they come from. Now we understand better that the i-process is somehow connected.”
Using ATLAS’s CARIBU source, scientists were able to study which barium isotopes capture neutrons to eventually form lanthanum, a byproduct of the decay of barium-139 and a key indicator of the i-process. However, determining this neutron capture rate is particularly difficult because the half-life of barium-139 is only 83 minutes.
Using experimental techniques, the researchers discovered that it was possible to indirectly determine this rate using a beam of the isotope cesium-140. This isotope undergoes radioactive decay into barium-140 and thus emits a gamma ray, which the researchers were able to detect and measure using the FRIB’s Summing Nal (SuN) detector, a total absorption gamma-ray spectrometer. By capturing the data of this process more precisely, the researchers were able to indirectly calculate the reaction rate of barium-139 as it transforms into barium-140, as well as the probability that this reaction will produce lanthanum.
“The technique used requires radioactive beams that are both fairly high in intensity and very pure,” said ATLAS Director Guy Savard, an Argonne Distinguished Fellow. “CARIBU provides these conditions for a range of neutron-rich isotopes.”
Armed with this new knowledge, the researchers can apply what they discovered in this study to other use cases at CARIBU and its upcoming upgrade, nuCARIBU. This will allow them to deepen their understanding of how neutron capture works for neutron-rich isotopes in the i-process. They ultimately hope to find a more direct way to study the process.
“In the fall, we will launch a large experimental campaign with nuCARIBU, which will allow us to take new measurements to expand the range of applications of this technique, to examine many cases and to try to understand how this neutron capture works on neutron-rich isotopes,” said Savard. “This is just a first step,” he added.
French Besides Spyrou, Mücher and Savard, authors include PA Denissenkov, F. Herwig, EC Good, G. Balk, HC Berg, DL Bleuel, JA Clark, C. Dembski, PA DeYoung, B. Greaves, M. Guttormsen, C Harris, AC Larsen, SN Liddick, S Lyons, M Markova, MJ Mogannam, S Nikas, J Owens-Fryar, A Palmisano-Kyle, G Perdikakis, F Pogliano, M Quintieri, AL Richard , D. Santiago-Gonzalez, MK Smith, A. Sweet, A. Tsantiri, and M. Wiedeking.
More information:
A. Spyrou et al, First study of the 139Ba(n,γ)140Ba Reaction to constrain the conditions of the astrophysical process i, Physical Exam Letters (2024). DOI: 10.1103/PhysRevLett.132.202701
Provided by Argonne National Laboratory
Quote:Experimental data help unravel the mystery surrounding the creation of heavy elements in stars (2024, September 12) retrieved September 12, 2024 from
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without written permission. The content is provided for informational purposes only.