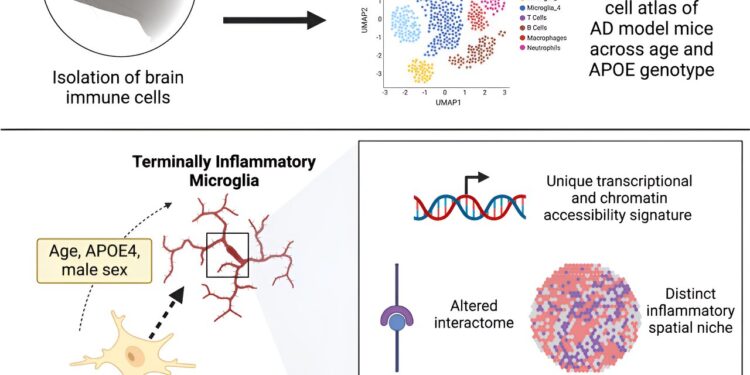
Graphical summary. Credit: Immunity (2023). DOI: 10.1016/j.immuni.2023.12.001
Mice reach the twilight of their lives around the age of two, the approximate equivalent of 80 years in humans. And when researchers introduce specific mutations into mice and age them, the mice can become distracted and irritable, eventually exhibiting signs of Alzheimer’s disease not unlike those of many elderly humans.
Now, a study published in the journal Immunitydemonstrates that microglia, the brain’s immune cells, die off as Alzheimer’s disease takes hold in mice and humans, and that APOE4, a key genetic variant implicated in Alzheimer’s disease, can mediate these changes.
“Older mice and those carrying the APOE4 variant have exhausted and tired immune cells in their brains, and we discovered a similar phenomenon in human datasets,” says Sohail Tavazoie, Leon Hess Professor at Rockefeller. The team named this new class of exhausted cells TIM, for terminally ill inflammatory microglia. TIMs have lost the ability to effectively remove plaque from the brain and may thus contribute to Alzheimer’s disease.
The study also sheds light on how the Alzheimer’s disease drug aducanumab might interact with immune cells in the brain. “When mice carrying the APOE4 variant were treated with aducanumab, we observed that their TIM regained certain functionalities,” explains Alon Millet, graduate researcher at the Tavazoie laboratory.
Age and inflammation
Humans carry one of three variants of the APOE gene: APOE2, APOE3, and APOE4. Previous work from the Tavazoie lab has demonstrated that these variants may play a central role in how the body responds to diseases ranging from cancer to COVID, but the link between Alzheimer’s disease and APOE4 is particularly well established: the variant APOE4, carried by about 20 percent of the population, is considered one of the most important genetic risk factors for Alzheimer’s disease.
Tavazoie, Millet, and Jose Ledo (now a faculty member at the Medical University of South Carolina) spent four years developing mouse models of Alzheimer’s disease that express variants of human APOE, and then age to get a better idea of how APOE4 influences their brains. Alzheimer’s disease sets in. “Systematically generating these mice was a major undertaking,” says Tavazoie. “This was an ongoing project made possible by the coming together of Jose and Alon’s specific expertise.”
The team then constructed a single-cell atlas of brain immune cells from these mice and identified a population of microglia riddled with signs of stress and inflammation that had not been previously described.
The brains of mice with APOE4 were invaded by TIM, whereas other variants had comparatively less TIM. Once they knew what to look for, the team also began finding TIM in human brain tissue donated by patients with the APOE4 variant. The results suggest that APOE4 may increase the risk of Alzheimer’s disease by weakening immune cells in the brain.
The researchers also found that treating mice with aducanumab, a recently approved Alzheimer’s disease drug, improved their condition and rehabilitated damaged TIM. Interestingly, the effects of the drug were much more pronounced in mice with APOE4. And even if these preliminary results cannot be immediately translated into the clinic, “this could be a first clue that aducanumab acts differently with different genotypes”, explains Millet. “That’s something clinicians should look at.”
Helping the immune system help itself
Some researchers suspect that a healthy immune system removes plaque before it builds up in the brain and that Alzheimer’s disease is what happens when this system fails and plaque builds up. According to this theory, rehabilitating microglia that are too tired to do their job could give the brain the boost it needs to protect itself. If so, TIM would be a promising therapeutic target.
“TIMs marinate in this inflammatory environment for years until they are no longer able to cope with it,” explains Millet. “If we can bring them back to a healthy state, maybe the immune system will be able to control Alzheimer’s disease.”
On this front, the team will now explore the signaling molecules that lead to TIM formation, with the aim of contributing to the development of drugs that interfere with the process, keep microglia healthy and reduce cognitive decline. . In the long term, this could lead to a new therapy for Alzheimer’s disease.
The team will also test whether TIM exists in other diseases. Millet suspects that although TIMs may have gone unnoticed until now, these exhausted immune cells could also be involved in other brain diseases, from tumors to Parkinson’s disease. “Inflammation causes TIMs to accumulate, so maybe what we’re seeing is not specific to Alzheimer’s disease,” he says.
“Most microglia could become TIMs if we give them enough time.”
More information:
Alon Millet et al, An exhausted microglial population accumulates in aged and APOE4 Alzheimer’s genotype brains, Immunity (2023). DOI: 10.1016/j.immuni.2023.12.001
Provided by Rockefeller University
Quote: “Exhausted” immune cells could be the cause of Alzheimer’s disease (January 10, 2024) retrieved January 10, 2024 from
This document is subject to copyright. Apart from fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.