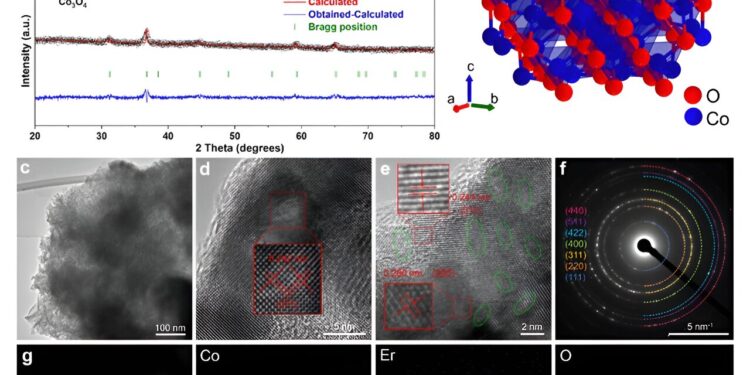
Structural characterizations of the as-prepared catalysts. (a) XRD patterns of Co3O4 and 4% Er−Co3O4. (b) Crystal structure of Co3O4 in this study. (c) TEM image of 4% Er−Co3O4(from) HRTEM images by (d) Co3O4 and (e) 4% Er− Co3O4 (the green ellipse represents the network faults). (f) SAED model of 4% Er− Co3O4. (g) Dark-field TEM image and corresponding elemental maps of Co, Er and O in 4% Er−Co3O4. Credit: ACS Catalysis (2024). DOI: 10.1021/acscatal.4c03088
A group of researchers has developed an electrocatalyst that promises to significantly improve the efficiency and stability of oxygen evolution reactions (OERs) in acidic environments. By incorporating a rare earth element, erbium (Er), into cobalt oxide (Co3O4) catalyst, the team demonstrated a new cost-effective solution that outperforms many non-precious metal catalysts, providing an alternative to more expensive noble metal-based options.
Details of the results were published in the journal ACS Catalysiswhere the study was also selected as an Editors’ Choice.
The oxygen evolution reaction, a critical process in water splitting and energy conversion, requires robust and efficient catalysts, especially under acidic conditions. Traditionally, cobalt-based catalysts have shown moderate performance due to their spinel crystal structure, which provides stability and catalytic activity. However, the introduction of Er into Co3O4 The catalyst marks a significant leap forward.
“Our research shows that by doping a small amount of erbium into Co3O4“We can improve both the activity and stability of the catalyst,” says Tianyi Wang, a JSPS postdoctoral researcher at Tohoku University’s Advanced Institute for Materials Research (WPI-AIMR) and co-author of the paper.
“Co doped with 4% erbium3O4 The catalyst we developed achieved an impressive overpotential of only 321 mV at 10 mA cm2 and maintained its stability for more than 250 hours. This performance is comparable to, and in some cases superior to, more expensive iridium-based catalysts.
Evaluation of OER electrochemical activity. (a) LSV curves of different catalysts at a scan rate of 1 mV/s and negative scan after iR correction. (b) Tafel diagrams of 4% Er-Co3O4ErCo3and Co.3O4. (c) EIS Nyquist plots of 4% Er-Co3O4ErCoO3 and Co3O4 at 1.333 V (defined potential). The inset shows the equivalent circuit (Rs: series resistance; Rct: charge transfer resistance). (d) TOF bar graph (based on current density at the corrected overvoltage iR of 430 mV). (e) Fit plots show Cdl values for 4% Er-Co3O4ErCoO3 and Co3O4. (f) Chronopotentiograms of 4% Er-Co3O4 and Co.3O4 at 10 mA cm-2. Credit: ACS Catalysis (2024). DOI: 10.1021/acscatal.4c03088
The team used advanced techniques, such as microkinetic modeling and density functional theory (DFT) calculations, to understand why this doped catalyst works so well. The incorporation of Er led to the formation of more active sites and defects in the crystal structure, which in turn increased the Co ratio3+ to Co2+ ions. This change plays a key role in generating oxygen vacancies, crucial for accelerating the OER process.
“Think of the catalyst as a road,” says Hao Li, an associate professor at WPI-AIMR and co-author of the paper. “Er doping essentially adds extra pathways, allowing more traffic to move smoothly. In this case, the traffic is the oxygen intermediates needed for the reaction. The increased number of oxygen vacancies acts as those extra pathways, speeding up the reaction.”
Furthermore, in situ Raman spectroscopy revealed that the oxygen vacancies created by Er doping were located in the octahedral sites of Co3O4 structure. These gaps have facilitated the development of critical intermediate species, which are essential to drive the OER process. The higher Co3+/Co2+⁺ The report further improved the catalytic activity by providing more active Co3+ sites to anchor these intermediaries.
Theoretical analyses of the active OER phase and the activity of erbium-doped cobalt3O4 surfaces. (a) Calculated 1D surface Pourbaix diagram. (b) Calculated 2D surface Pourbaix diagram. (c) Microkinetic OER volcanic activity model at 10 mA cm-2 as a function of GO-GHO. Theoretical predictions of FeNi-Ni, FeNi-Fe and CoO were obtained from existing literature. The inset shows the identified surface coverage of Er−Co3O4 (311) under OER operating conditions. Er, Co and O are shown in green, purple and red, respectively. Credit: ACS Catalysis (2024). DOI: 10.1021/acscatal.4c03088
“This combination of electronic and structural optimization is what really makes the catalyst special,” Wang adds. “It’s the balance between these two factors that allows us to achieve such high performance.”
This research opens new possibilities for the design of high-performance catalysts that are both cost-effective and sustainable. In the future, the researchers plan to focus more on non-precious metal doping to design high-performance, low-cost, and readily available electrocatalysts.
More information:
Sanjiang Pan et al, Erbium doping improves oxygen evolution performance of cobalt oxide in acidic medium, ACS Catalysis (2024). DOI: 10.1021/acscatal.4c03088
Provided by Tohoku University
Quote: Erbium-doped electrocatalyst enhances oxygen evolution reactions in acidic environments (2024, September 13) retrieved September 13, 2024 from
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without written permission. The content is provided for informational purposes only.