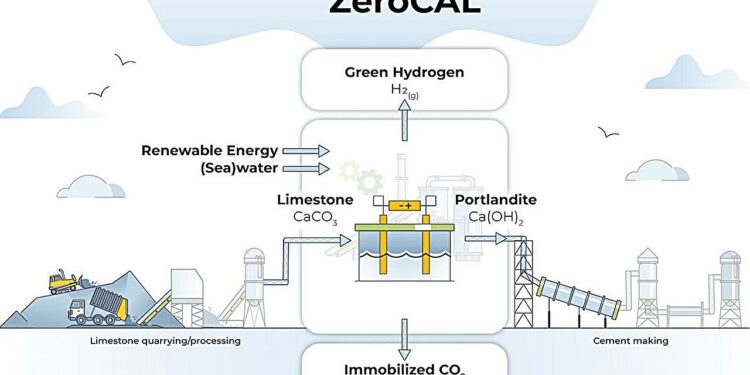
The ZeroCAL approach, which can be integrated into the existing cement production process, uses limestone as a feedstock to produce calcium hydroxide, which does not emit carbon dioxide when burned to produce lime for cement. Byproducts of the process include hydrogen gas, which can be used as a clean fuel to heat cement kilns. Credit: Adrienne Johnston, University of California, Los Angeles
Researchers at UCLA’s Institute for Carbon Management have developed a method that could eliminate almost all of the carbon dioxide emitted during the cement production process, which accounts for about 8% of global atmospheric CO.2 emissions.
In a new study published in ACS Sustainable Chemistry and EngineeringThe researchers describe how the new approach could be easily integrated into existing cement production processes, providing a more affordable alternative to existing solutions to decarbonize the industry.
Cement and concrete: a huge carbon footprint
Ordinary Portland cement, the most common type of cement, is a base material used as a binder for almost all modern concrete, the most widely used material in the world after water. This cement is made from limestone, an abundant and economical natural source of lime.
However, the traditional method of producing cement, which involves heating limestone in a fossil fuel-fired kiln to break its chemical bonds, leaves a huge carbon footprint, resulting in the emission of almost a kilogram of carbon dioxide per kilogram of cement produced.
The thermochemical decomposition of limestone to produce lime, or calcium oxide, the main precursor for cement production, represents around 60% of CO2 released, while the burning of fossil fuels to heat the furnace in which the chemical reaction takes place accounts for the remaining 40%.
This process also consumes a lot of energy. Producing one metric ton of lime (about 2,200 pounds) requires about 1.4 megawatt hours, enough to power one and a half American homes for an entire month.
The “ZeroCAL” solution: Eliminate CO2 broadcasts
Unlike limestone, calcium hydroxide, a carbon-free precursor for lime and cement production, emits only water when heated in a kiln to produce lime. Inspired by this, a team at UCLA’s Samueli School of Engineering led by Gaurav Sant, director of the Institute for Carbon Management, or ICM, and professor of civil and environmental engineering, developed a new approach to produce calcium hydroxide.
Using limestone as a raw material, the researchers first dissolved the limestone in a water-based solution containing a common industrial acid called ethylenediaminetetraacetic acid. Using membrane nanofiltration, they then separated the calcium derived from the limestone before using an electrochemical process to produce calcium hydroxide.
The researchers called their method “ZeroCAL,” which stands for carbon-free lime, and this approach, they say, could eliminate 98% of CO.2 emissions associated with the thermochemical process of creating lime. ZeroCAL’s byproducts include commonly used hydrochloric acid and baking soda, as well as oxygen and hydrogen gas, the latter of which can be used as a clean fuel to heat cement kilns.
“The ZeroCAL approach offers an elegant solution to eliminate carbon dioxide emissions associated with the cement production process,” said Sant, corresponding author of the study and the Pritzker Professor of Sustainability at UCLA Samueli.
“First, it solves carbon emissions resulting from decomposition of limestone while providing clean hydrogen and oxygen to heat the cement kiln. Second, it enables on-site decarbonization while using existing kilns and limestone feedstocks without having to build separate carbon capture and storage facilities. “.
Although the process currently requires more energy than existing lime production methods, ongoing research suggests avenues by which ZeroCAL can achieve energy consumption parity by simplifying and eliminating unit operations and making a better use of acidic and basic co-products produced by electrolysis.
To meet ZeroCAL’s water demand, the team suggests focusing on cement plants near coasts or rivers, or facilities with easy access to water supplies. The researchers said they are working with Ultratech Cement Limited, the largest cement manufacturer in India – the world’s second-largest cement market – to build a first-of-its-kind demonstration plant that will produce one metric ton of lime per day using the ZeroCAL Process.
“It has become very clear that mitigating climate change requires urgent and revolutionary actions in many areas to decarbonize our society,” said Fabian Rosner, study co-author and assistant professor of civil and environmental engineering at UCLA Samueli.
“We believe the ZeroCAL process offers a unique pathway to enable accessible and rapidly scalable decarbonization of cement production in a way we have not previously considered.
The ZeroCAL solution could also open a new path to decarbonizing steel production by leveraging low-carbon lime as a source of calcium in the manufacturing process, the researchers said.
Besides Sant, the ICM study’s authors include first author Adriano Leão and ICM associate directors David Jassby, professor of civil and environmental engineering at UCLA Samueli, and Dante Simonetti, associate professor of chemical engineering and biomolecular at UCLA Samueli.
More information:
Adriano Leão et al, ZeroCAL: Eliminating carbon dioxide emissions linked to limestone decomposition to decarbonize cement production, ACS Sustainable Chemistry and Engineering (2024). DOI: 10.1021/acssuschemeng.4c03193
Provided by University of California, Los Angeles
Quote: Engineers develop a scalable process to decarbonize cement production (October 14, 2024) retrieved October 14, 2024 from
This document is subject to copyright. Except for fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for informational purposes only.