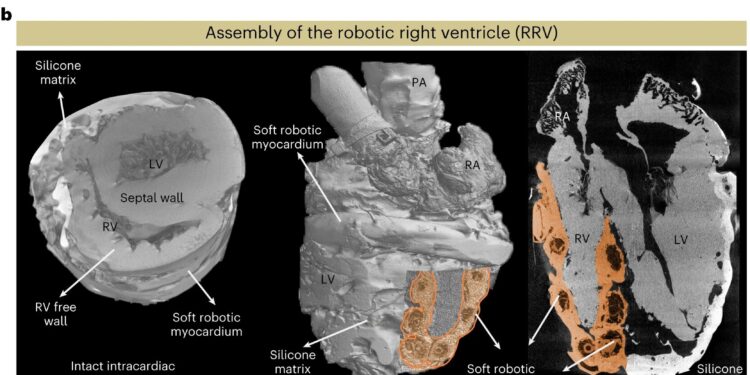
An overview of the RRV and its architecture. a, Overview of the bioinspired and biomimetic approach used to create a right-beating biohybrid heart. Initially, a freshly explanted porcine heart underwent chemical treatment, after which the native myocardium was dissected by hand and replaced with a robotic counterpart while preserving the endocardial scaffold. b, The RRV assembly was imaged by micro-CT, revealing the preserved intracardiac structures. c, Schematic of the complex RV shape, fiber orientation, and wall movement (left). The image of the heart showing the orientation of fibers in the basal and apical loops is reprinted from Buckberg et al., with permission from Elsevier. The physical model of the soft robotic myocardium shows the placement of individual actuators in the synthetic myocardium (right). The RV outflow tract contains infundibular muscles that also contribute to RV ejection by contraction. Contraction of the flow region can be achieved by placing a circumferential actuator across the infundibulum. The numbers indicate the respective actuator location corresponding to the RV movement shown in the diagrams on the left. SVC, superior vena cava. Credit: Nature Cardiovascular Research (2023). DOI: 10.1038/s44161-023-00387-8
MIT engineers have developed a robotic replica of the heart’s right ventricle, which mimics the beating and blood pumping action of living hearts.
The ventricle robot combines real heart tissue with synthetic artificial muscles resembling balloons that allow scientists to control the contractions of the ventricle while observing the functioning of its natural valves and other complex structures.
The artificial ventricle can be adjusted to mimic healthy and diseased states. The team manipulated the model to simulate conditions of right ventricular dysfunction, including pulmonary hypertension and myocardial infarction. They also used the model to test cardiac devices. For example, the team implanted a mechanical valve to repair a faulty natural valve, then observed how the ventricle’s pumping changed in response.
They say the new robotic right ventricle, or RRV, can be used as a realistic platform to study right ventricular disorders and test devices and therapies aimed at treating these disorders.
“The right ventricle is particularly susceptible to dysfunction in intensive care units, especially in patients on mechanical ventilation,” says Manisha Singh, a postdoctoral fellow at MIT’s Institute of Medical Engineering and Sciences (IMES). “The RRV simulator may be used in the future to study the effects of mechanical ventilation on the right ventricle and to develop strategies to prevent right heart failure in these vulnerable patients.”
Singh and his colleagues report details of the new design in a paper appearing today in Nature Cardiovascular Research.
A ballet of rhythms
The right ventricle is one of the four chambers of the heart, along with the left ventricle and the left and right atria. Of the four chambers, the left ventricle lifts the heaviest, because its thick, cone-shaped musculature is designed to pump blood throughout the body. The right ventricle, Roche says, is a “ballerina” in comparison, because it carries a lighter but no less crucial load.
“The right ventricle pumps deoxygenated blood to the lungs, so it doesn’t need to pump as hard,” notes Roche. “It’s a finer muscle, with more complex architecture and movements.”
This anatomical complexity has made it difficult for clinicians to accurately observe and assess right ventricular function in patients with heart disease.
“Conventional tools often fail to capture the complex mechanics and dynamics of the right ventricle, leading to potential misdiagnoses and inadequate treatment strategies,” says Singh.
To improve understanding of this lesser-known chamber and accelerate the development of cardiac devices to treat its dysfunction, the team designed a realistic, functional model of the right ventricle that captures its anatomical intricacies and replicates its pumping function.
The model includes real heart tissue, which the team chose to incorporate because it retains natural structures too complex to reproduce synthetically.
“There are tiny chords and valve leaflets with different material properties that all move in concert with the muscle of the ventricle. Trying to mold or print these very delicate structures is a real challenge,” says Roche.
The shelf life of a heart
In the new study, the team reports the explantation of a pig’s right ventricle, which they treated to carefully preserve its internal structures.
They then placed a silicone wrap around it, which acted like a soft synthetic myocardium or muscle lining. Into this liner, the team embedded several long, balloon-shaped tubes, which encircled the actual heart tissue, in positions that the team determined through computer modeling to be optimal for replicating the contractions of the ventricle.
The researchers connected each tube to a control system, which they then set up to inflate and deflate each tube at rates that mimicked the actual rhythm and movement of the heart.
To test its pumping ability, the team perfused the model with a liquid of similar viscosity to blood. This particular liquid was also transparent, allowing engineers to observe with an internal camera how the valves and internal structures reacted as the ventricle pumped the liquid.
They found that the pumping power of the artificial ventricle and the function of its internal structures were similar to those previously observed in living, healthy animals, demonstrating that the model can realistically simulate the action and anatomy of the right ventricle. The researchers were also able to adjust the frequency and power of the pumping tubes to mimic various heart conditions, such as irregular heartbeats, muscle weakening, and hypertension.
“We are resuscitating the heart, in a sense, and in a way that we can study and potentially treat its dysfunction,” says Roche.
To show that the artificial ventricle can be used to test heart devices, the team surgically implanted ring-shaped medical devices of varying sizes to repair the chamber’s tricuspid valve, a one-way leafy valve that lets in the blood in the right ventricle. When this valve leaks or is physically compromised, it can cause right heart failure or atrial fibrillation and lead to symptoms such as reduced exercise capacity, swelling of the legs and abdomen, and an enlarged liver.
The researchers surgically manipulated the valve of the robot ventricle to simulate this condition, then replaced it by implanting a mechanical valve or repaired it using ring-shaped devices of different sizes. They observed which device improved fluid flow from the ventricle as it continued to pump.
“With its ability to accurately reproduce tricuspid valve dysfunction, RRV provides an ideal training ground for surgeons and interventional cardiologists,” says Singh. “They can practice new surgical techniques to repair or replace the tricuspid valve on our model before applying them to real patients.”
Currently, the RRV can simulate realistic functions over a few months. The team is working to extend this performance and enable the model to run continuously over longer periods of time. They also work with implantable device designers to test their prototypes on the artificial ventricle and potentially accelerate their path to patients. And in the longer term, Roche plans to combine RRV with a similar artificial and functional model of the left ventricle, which the group is currently refining.
“We plan to combine it with the left ventricle to create a fully adjustable artificial heart that could potentially work in humans,” says Roche. “We’re still a long way off, but that’s the big picture.”
More information:
Manisha Singh et al, The robotic right ventricle is a biohybrid platform that simulates right ventricular function under (patho)physiological conditions and interventions, Nature Cardiovascular Research (2023). DOI: 10.1038/s44161-023-00387-8
Provided by the Massachusetts Institute of Technology
This story is republished courtesy of MIT News (web.mit.edu/newsoffice/), a popular site that covers news about MIT research, innovation and education.
Quote: Engineers design a robotic replica of the right chamber of the heart (December 8, 2023) retrieved December 8, 2023 from
This document is subject to copyright. Except for fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.