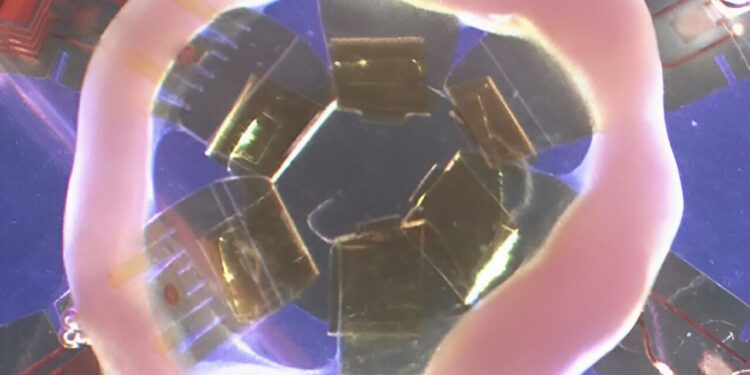
The engineered heart tissues were grown in rings to better mimic living human hearts. Credit: Elizabeth McNally, MD, PhD.
Northwestern Medicine scientists have developed a new method to measure cardiac contraction and electrical activity in engineered human heart tissue, according to findings published in Scientific progress.
For years, heart research has been limited by a lack of cellular models and has relied on animal models to replace human hearts, said Elizabeth McNally, MD, PhD, the Elizabeth J. Ward Professor of Genetic Medicine, who was co-senior author of the study.
“We need cells that actually beat and have electrical properties,” says McNally, who also directs the Center for Genetic Medicine. “We can take blood cells from patients with genetic diseases and turn them into stem cells in the petri dish. From those stem cells, we build three-dimensional artificial heart tissues that we can now monitor closely. These models have many of the properties of the human heart.”
For the study, McNally and his team of clinicians, geneticists and bioengineers created human heart tissue in a petri dish using cardiomyocytes derived from induced pluripotent stem cells collected from patients with genetic heart diseases. Normally, these cells are grown in flat dishes, but the researchers grew the cells in rings to better mimic living human hearts.
The scientists then attached the artificial hearts to tiny, flexible sensors that recorded muscle and electrical activity in real time. The sensors were developed in collaboration with John A. Rogers, Ph.D., the Louis Simpson and Kimberly Querrey Professor of Materials Science and Engineering, Biomedical Engineering and Neurological Surgery, who was co-senior author of the study, and Igor Efimov, Ph.D., professor of biomedical engineering and medicine in the Division of Cardiology.
Finally, the researchers induced irregular heartbeats in the cells and tested how the tissues responded to drugs normally used in patients to stabilize the heart rhythm. Two types of commonly used cardiac drugs were tested, and both returned the tissues’ heart rhythms to normal, the study found.
“It has been difficult to properly characterize the electrical properties of these types of tissues,” said Dominic Fullenkamp, MD ’14, PhD ’12, GME ’16-21, assistant professor of medicine in the division of cardiology and first author of the study.
“This platform can tell us about the propensity of patients to develop arrhythmias. It can allow us to perform an ECG on the tissues themselves, in the same way that we interrogate the electoral properties of the patient’s heart in the clinic. That’s the real advantage.”
In addition to allowing clinicians to study individualized models of their patients’ hearts, the ability to monitor function in engineered tissues can accelerate cardiac research more generally, McNally said.
“The next step is to figure out how to make some of these little devices so that other people in the field can use them as well,” McNally said.
Using engineered heart tissue could reduce the need for animal models in cardiac research, McNally said, and provide a human model for living hearts.
“If we get results from tissues that predict what happens in humans, that will be extremely valuable to us,” McNally said.
“We have a long history of using animal models in our field, but they are not predictive of what will happen in humans. This could reduce the amount of work that needs to be done in animals. We think using human cells is a good first step to achieve this.”
More information:
Dominic E. Fullenkamp et al., Simultaneous electromechanical monitoring in artificial heart tissue using a mesoscopic frame, Scientific progress (2024). DOI: 10.1126/sciadv.ado7089
Provided by Northwestern University
Quote: Engineering human heart tissue for scientific study (2024, September 26) retrieved September 26, 2024 from
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without written permission. The content is provided for informational purposes only.