Credit: Journal of the American Chemical Society (2024). DOI: 10.1021/jacs.4c08518
MXenes are a class of two-dimensional materials discovered in 2011. Theoretical studies previously predicted that they would not be catalytically active in anodic processes. Researchers led by Professor Kai S. Exner, head of the Department of Theoretical Catalysis and Electrochemistry at the University of Duisburg-Essen (UDE), refuted this theory using multi-scale modeling.
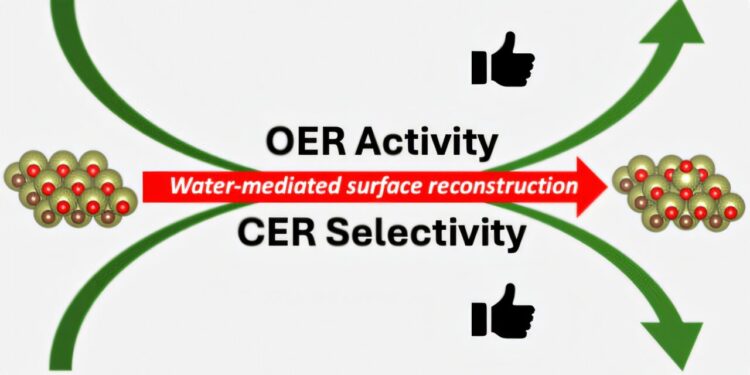
The scientists found that when an electrode potential is applied, the surface of MXene transforms into a brush-like structure. The non-noble metal atoms migrate and form what are called “SAC-like structures” (similar to a single-atom catalyst). These catalysts are involved in two important reactions, namely the oxygen evolution and chlorine evolution reactions.
The result is a material whose surface has catalytically active sites without the addition of precious metals. “We concluded that MXenes behave similarly to enzymes in an electrochemical environment. By applying an electrode potential, their active sites are created directly during the process,” explains Exner. The research is published in the Journal of the American Chemical Society.
The team was also able to show that the resulting SAC-like structures are selective: if water and chloride ions are simultaneously in the reaction environment, only chlorine gas is formed. The formation of this basic chemical is a key process in the chemical industry, which produces more than 70 million tons of gaseous chlorine (Cl2) per year. Cl2 is necessary for the production of pharmaceuticals, plastics, batteries and water treatment.
However, when only water is available in the electrolyte, the active surface area of MXene facilitates the production of oxygen gas (O2) by means of the release of oxygen, an important step in the formation of green hydrogen in an electrolyzer.
This discovery can greatly simplify the production of single-atom catalysts. Eliminating expensive precious metals also reduces costs and dependencies.
More information:
Samad Razzaq et al, MXenes spontaneously form active and selective single-atom centers under anodic polarization conditions, Journal of the American Chemical Society (2024). DOI: 10.1021/jacs.4c08518
Provided by the University of Duisburg-Essen
Quote: Evolution of oxygen and chlorine without noble metals: the potential of the electrode transforms MXene surfaces (2024, December 17) retrieved on December 17, 2024 from
This document is subject to copyright. Except for fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for informational purposes only.