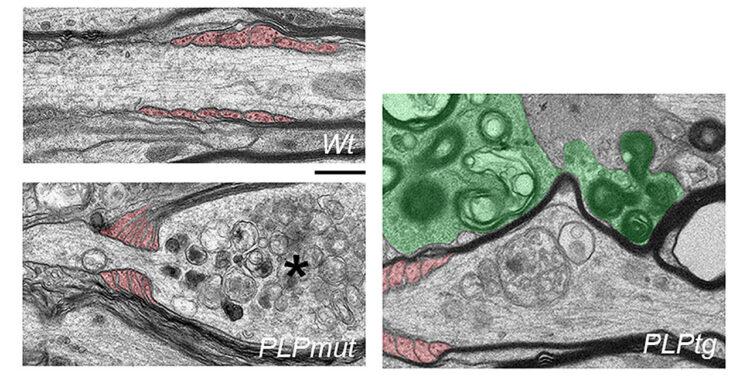
Electron microscope images of white matter axons in healthy control mice (top) and mice with different myelin gene defects. Axons that remain wrapped in abnormal myelin (middle) are constricted by distal oligodendrocyte processes (colored red) and show signs of degeneration (asterisk). Removal of abnormal myelin (bottom) by microglia (colored green) increases the chances of axon survival. Scale bar: 0.5 µm. Credit: Natural communications (2023). DOI: 10.1038/s41467-023-42570-2
Myelin is an insulating sheath around axons (the processes connecting nerve cells) that is primarily composed of lipids and proteins. It allows rapid conduction of electrical signals and supports neuronal integrity and function. In the central nervous system, myelin is formed from specialized glial cells called oligodendrocytes.
Myelinated fiber bundles are particularly vulnerable to various pathogenic processes, and myelin diseases are often associated with chronic inflammation of the nervous system. A good example is multiple sclerosis, a serious and common neurological disease in which immune cells cause demyelination, or loss of myelin. However, maladaptive immune responses also contribute to other disorders associated with myelin abnormalities, including hereditary and aging-related diseases.
It is important to note that degeneration of axons and neurons is a major determinant of the clinical severity of these disorders. It is generally accepted that loss of myelin results in increased vulnerability of denuded axons to a toxic inflammatory milieu and ultimately results in their demise.
This purely detrimental view of demyelination is challenged by a recent study conducted at the Department of Neurology under the direction of corresponding author and lecturer Dr. Janos Groh from the Section of Developmental Neurobiology (Prof. Dr. Rudolf Martini, Hospital University of Würzburg) and in collaboration with the Institute for Molecular Neurobiology (Prof. Dr. Mikael Simons, Technical University of Munich).
Supported by Professor Antoine-Emmanuel Saliba from the Helmholtz Institute for Research on RNA Infections (Würzburg) and teams of researchers from Hanover and Cambridge, they have just published the results of their study in the journal Natural communications.
Myelin gene defects trigger distinct immune responses
To study the relationship between myelin loss and axon degeneration, researchers studied mouse models of rare diseases carrying defects in the central nervous system’s main myelin protein. “These rare monogenetic disease models offer unique opportunities to reveal mechanisms that are broadly relevant to much more common disorders,” explains Rudolf Martini.
Scientists had previously discovered that the formation of abnormal or “bad” myelin in these mice led to an inflammatory response including an accumulation of cytotoxic CD8 cells.+ T cells. In the disease models analyzed, these adaptive immune cells target and damage fiber segments with abnormal myelin, reminiscent of multiple sclerosis.
Surprisingly and contrary to prevailing opinion, they discovered an inverse relationship between axon loss and demyelination when comparing disease models. Fibers that remained myelinated despite chronic T cell attack were at higher risk of degenerating, while those that lost myelin survived. Furthermore, the behavioral deficits of the mice were more clearly correlated with neurodegeneration than with demyelination.
Persistence of abnormal myelin as a risk factor for axon degeneration
“This inverse relationship was unexpected and prompted us to study in more detail the interactions between abnormal oligodendrocytes and another type of immune cell called microglia,” explains Janos Groh. Microglia are cells of the innate immune system that populate the central nervous system and can orchestrate harmful and beneficial immune responses.
In their study, the authors used different pharmacological approaches to modulate the elimination of abnormal myelin by microglia. “We show that efficient removal of disrupted myelin by microglia under adaptive immune attack allows survival of axons at stages of reversible damage,” adds Groh. Thus, persistent coating with “bad” myelin appears to be worse for neurons than loss of myelin, at least when myelin is targeted by adaptive immunity.
Oligodendrocytes under immune attack actively harm axons
Scientists could also identify a mechanism by which oligodendrocytes attacked by T cells harm their axonal partners. They discovered an aberrant constriction response in the myelinating processes wrapped around axons. “When we inhibited this aberrant constriction by paralyzing the cytoskeletal filaments, we were able to reduce axon degeneration,” says Groh.
“The T-cell attack seemed to trigger the oligodendrocytes to strangle the axons like a constrictor snake,” Martini adds.
“What is the biological significance of these highly organized but self-harming processes?” Martini said. Researchers believe these oligodendrocyte reactions may be beneficial in other conditions, such as nervous system damage. However, aberrant induction of these immune mechanisms could constitute a detrimental response in many diseases.
According to Groh and Martini, their study identified potential targets for the treatment of diseases associated with myelin abnormalities and inflammation of the nervous system. Furthermore, they emphasize that new therapeutic approaches for myelin diseases should ideally block harmful immune responses while allowing beneficial immune responses, such as the elimination of “bad” myelin. This could help promote neuronal resilience mechanisms, a prerequisite for healing nervous system damage.
More information:
Janos Groh et al, Microglia-mediated demyelination protects against CD8+ T cell-induced axon degeneration in mice with PLP defects, Natural communications (2023). DOI: 10.1038/s41467-023-42570-2
Provided by Julius-Maximilians-Universität Würzburg
Quote: Effective removal of abnormal myelin allows survival of nerve fibers targeted by adaptive immune cells (November 28, 2023) retrieved November 28, 2023 from
This document is subject to copyright. Except for fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.