Immunogenomic correlates and clinical outcomes with PCP chemoimmunotherapy in patients with STK11 and/or KEAP1 mutant advanced NSCLC. Credit: Nature (2024). DOI: 10.1038/s41586-024-07943-7
Researchers at the University of Texas MD Anderson Cancer Center have demonstrated that patients with metastatic non-squamous non-small cell lung cancer (NSCLC) harbor specific mutations in the tumor suppressor genes STK11 and/or KEAP1 were more likely to benefit from the addition of immunotherapy. tremelimumab to a combination of durvalumab and chemotherapy to overcome treatment resistance typically seen in this patient population.
Results of the study, published in Natureidentify KEAP1 and STK11 as potential biomarkers to stratify patients most likely to benefit from the addition of CTLA-4 immune checkpoint inhibitors, including tremelimumab.
In these patients, the addition of tremelimumab to durvalumab and chemotherapy resulted in higher overall response rates (42.9%) compared to patients who received durvalumab plus chemotherapy (30.2%) or chemotherapy alone (28%). These results were further supported by preclinical models, supporting the use of dual checkpoint inhibitors for patients with these mutations.
“STK11 and KEAP1 alterations are common in patients with NSCLC and are linked to poor clinical outcomes with current first-line treatments,” said co-senior author Ferdinandos Skoulidis, MD, Ph.D., professor thoracic aggregate. /Medical oncology of the head and neck.
“While previous research suggested potential benefits of adding CTLA-4 inhibitors to PD-1 or PD-L1 inhibitors, we have no reliable biomarkers to predict which patients would have the best outcomes. This study provides the strongest evidence to date that patients with STK11 and/or KEAP1-mutated NSCLC can selectively benefit from dual immune checkpoint inhibition.
This research study, made possible through collaboration between 22 academic centers in North America and Europe as well as biotechnology and pharmaceutical companies, combines analyzes of clinical cohorts, patient samples, laboratory models and data from the POSEIDON phase III clinical trial.
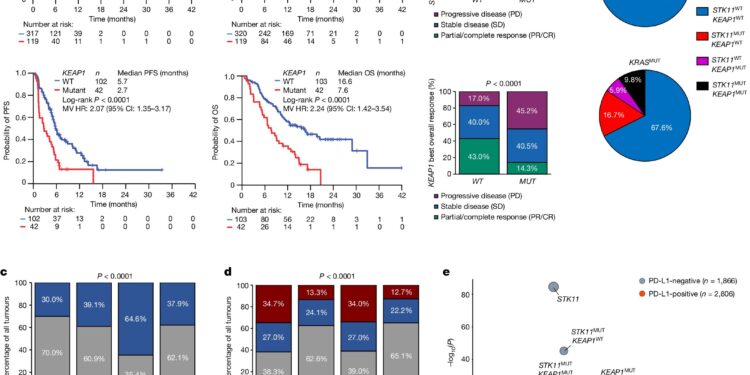
Initial observations in a clinical cohort of 871 NSCLC patients demonstrated that patients with STK11 and/or KEAP1 alterations had poorer outcomes with chemotherapy plus the PD-1 inhibitor pembrolizumab. The researchers then studied the immune and genetic characteristics of 8,592 non-squamous NSCLC tumors.
They found that mutations in the STK11 and KEAP1 genes were linked to a less favorable tumor environment, often referred to as a “cold” microenvironment. This type of environment contained many myeloid suppressor cells and fewer CD8+ Cytotoxic T cells, important for fighting tumors. However, they noted that CD4+ immune cells were less affected and remained present in tumors with STK11 and/or KEAP1 mutations.
Based on these observations, researchers hypothesized that dual checkpoint inhibitors, targeting CTLA-4 in addition to PD-1 or PD-L1, might improve outcomes. In an analysis of 1,013 patients from the POSEIDON study, researchers confirmed that tremelimumab plus durvalumab and chemotherapy improved response rates, progression-free survival, and overall survival.
To further explore these findings, the experts then evaluated the effects of single and dual immune checkpoint inhibition on the tumor microenvironment in several preclinical models of STK11 and/or KEAP1-mutated NSCLC. Compared to PD-1 inhibition alone, dual checkpoint blockade strongly improved the tumor microenvironment by increasing the presence of specific immune cells that stimulate the antitumor response, providing a possible mechanism that may explain the observed benefits.
“These results confirm that NSCLC patients with STK11 or KEAP1 mutations are relatively resistant to standard combinations of PD-(L)1 inhibitors and chemotherapy, but may experience notable benefit when a CTLA-4 inhibitor is added to their treatment regimen,” said co-senior author John Heymach, MD, PhD, chairman of Thoracic/Head and Neck Medical Oncology.
“We are optimistic that these results will encourage clinicians to consider this new therapeutic approach as a preferred treatment option.”
Limitations of this study include the fact that some of the results were analyzed after study completion, as well as the limited number of patients with STK11 and/or KEAP1 alterations. In the ongoing Phase IIIB TRITON trial, investigators will prospectively compare dual checkpoint blockade with durvalumab and tremelimumab versus pembrolizumab in combination with chemotherapy in patients with advanced non-squamous NSCLC with STK11 alterations , KEAP1 or KRAS.
More information:
Ferdinandos Skoulidis et al, CTLA4 blockade abrogates KEAP1/STK11-related resistance to PD-(L)1 inhibitors, Nature (2024). DOI: 10.1038/s41586-024-07943-7
Provided by the University of Texas MD Anderson Cancer Center
Quote: Double immunotherapy plus chemotherapy shown to be beneficial for a specific subset of lung cancer patients (October 11, 2024) retrieved October 11, 2024 from
This document is subject to copyright. Except for fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for informational purposes only.