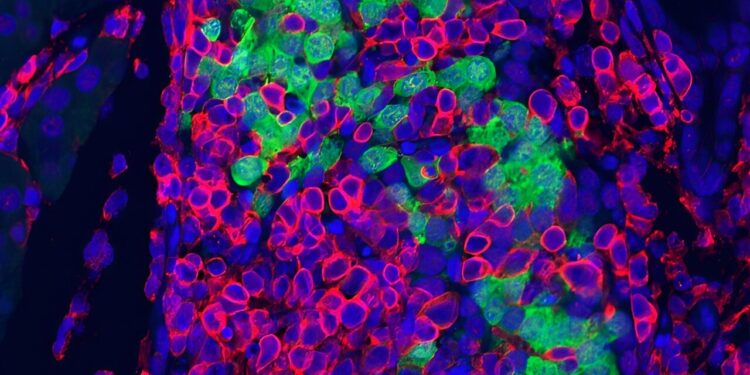
Image of a mouse pancreas with disrupted RNA editing in beta cells, showing massive inflammation destroying an islet of Langerhans. Blue, DNA; Insulin-producing green beta cells; red and immune cells. Credit: Dor Lab
A recent study published in Cellular metabolism by researchers from Hebrew University-Hadassah Medical School, Bar-Ilan University and Vanderbilt University, developed a new paradigm for early stages of type 1 diabetes (T1D), suggesting a new etiology that does not involve viral infection.
T1D is an autoimmune disease that affects nearly 10 million people worldwide in which the immune system attacks and destroys the insulin-producing beta cells of the pancreas. In the absence of insulin, the concentration of glucose in the blood increases, leading to many complications. Patients, usually diagnosed in childhood, require lifelong insulin treatment.
One advanced model for why T1D develops suggests that the disease is initiated by a viral infection, which in genetically susceptible individuals causes an autoimmune attack on beta cells. This is supported by much information, for example the identification of an antiviral response at an early stage of the disease.
The implications of this view are vast; for example, he suggests the use of antiviral treatment to prevent T1D. However, despite decades of research, no causal virus has been found.
The new research, led by Professor Yuval Dor, Dr. Agnes Klochendler and MD/Ph.D. Students Ehud Knebel and Shani Peleg present a new model for how T1D can develop, explaining the antiviral response but without requiring viral infection.
The team studied a process called RNA editing, which works to dismantle endogenous RNA molecules that fold back on themselves, forming double-stranded RNA. Since double-stranded RNA is a hallmark of many viruses, these molecules can often be misrecognized by the immune system as an indication of an invading virus and trigger a harmful immune response.
Researchers have discovered that when RNA editing is defective in the beta cells of the pancreas, the body launches a massive inflammatory attack, destroying the beta cells and ultimately leading to diabetes, with features that strikingly resemble T1D.
Additionally, they found that high blood glucose levels amplified the inflammatory attack, suggesting a vicious cycle in which beta cell destruction leads to diabetes, which results in destructive inflammation. Surprisingly, independent work has recently found that genetically inherited defects in RNA editing predispose people to multiple autoinflammatory diseases, including T1D, suggesting relevance to actual human T1D.
Professor Yuval Dor said: “Our research presents compelling evidence that disruption of RNA editing in beta cells can trigger an inflammatory response resembling type 1 diabetes at an early stage. This offers new insight into how T1D may develop, with implications for prevention and treatment. strategies.”
Dr Agnes Klochendler added: “Identifying a link between natural double-stranded RNA in beta cells, inflammation and diabetes opens a new perspective on T1D: an ‘enemy within’ paradigm , not requiring external viral infection as a triggering event for this disease. “.
More information:
Udi Ehud Knebel et al, Disrupted RNA editing in beta cells mimics early-stage type 1 diabetes, Cellular metabolism (2023). DOI: 10.1016/j.cmet.2023.11.011
Provided by the Hebrew University of Jerusalem
Quote: New model of type 1 diabetes: disruption of RNA editing mimics early-stage disease without virus involvement (December 27, 2023) retrieved December 27, 2023 from
This document is subject to copyright. Apart from fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.