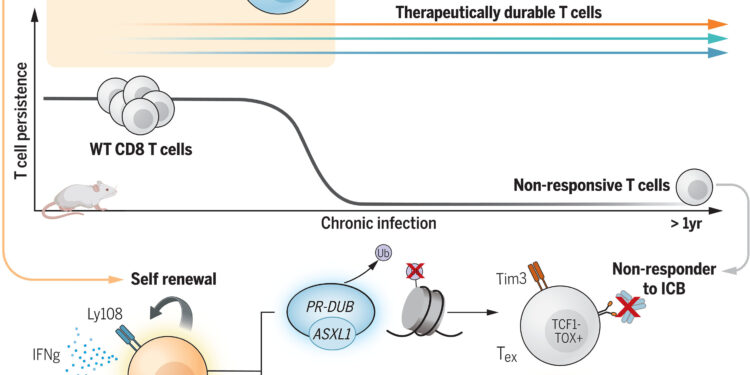
Core epigenetic regulators in maintaining therapeutic and sustainable multipotent Tpex populations. Credit: Science (2024). DOI: 10.1126/science.adl4492
Immunotherapy, which uses the patient’s own immune system to treat disease, has shown promise in some cancer patients but has not worked in most. New research from St. Jude Children’s Research Hospital and colleagues found that disrupting Asxl1, a T cell gene, improved sensitivity to a type of immunotherapy called immune checkpoint blockade and improved control of long-term tumors in model systems. The results appear in Science.
Immune system cells use “checkpoints” or signals that tell them how to respond to diseased cells or pathogens. Tumors can hijack these checkpoints to disable the immune system, helping cancer cells hide and survive. Immune checkpoint inhibitors or blockades can stop the suppressive effects of tumors, helping the immune system detect and kill cancer cells.
“We found that disrupting the Axsl1 gene in T cells led to an enhanced response to immune checkpoint blockade,” said co-senior corresponding author Caitlin Zebley, MD, Ph.D., Department of Transplantation of Bone Marrow and Cellular Therapy at St. Jude.
T cells that encounter too many tumor cell fragments can also become exhausted and lose their ability to kill cancer cells. The researchers showed that eliminating Asxl1 prevented T cell exhaustion, enabling long-term immune responses.
“We discovered that Asxl1 controls the epigenetic checkpoint that enhances terminal differentiation of T cells to the exhausted state. When T cells differentiate beyond this checkpoint, they become essentially useless for l “immunotherapy,” said co-corresponding author Ben Youngblood, Ph.D., Department of Immunology at St. Jude. “Our discovery of this molecular checkpoint represents a crucial advance in this field, as it now allows us to develop more T cells with a durable anti-tumor response.”
Achieving this discovery required expertise in immune cell signaling and immunotherapy, as well as samples from patients successfully treated with immune checkpoint blockade, highlighting the importance of collaboration in scientific research.
“Immunotherapies have saved countless lives. Today’s results demonstrate how epigenetics can further improve these powerful treatments to help even more people,” said co-author Peter A. Jones, Ph.D. ., D.Sc. (honorable), President and Scientific Director of the Van Andel Institute and co-leader of the Van Andel Institute Dream Team – Stand Up To Cancer Epigenetics, which provided data on patients who received immunotherapy. “We are delighted to support this vital work, which highlights the immense power of collaboration as we fight cancer together.”
(Left to right) First author Tae Gun Kang, Ph.D., Department of Immunology at St. Jude, co-senior corresponding author Caitlin Zebley, MD, Ph.D., Department of Bone Marrow Transplantation and Therapy St. Jude Cellular and co-corresponding author Ben Youngblood, Ph.D., St. Jude Department of Immunology. Credit: Courtesy of St. Jude Children’s Research Hospital
Reverse engineering immunotherapy success stories
Immune checkpoint blockade has been shown to be highly effective and sometimes curative in a subset of cancer patients, with its discovery earning James P. Allison and Tasuku Honjo the 2018 Nobel Prize in Physiology or Medicine. But this approach does not work for all patients. Youngblood, Zebley and their colleagues therefore examined the genetics of responders to determine what is different in the biology of individuals who respond to immune checkpoint blockade.
“We looked at a small cohort of patients with myelodysplastic syndrome who had significantly improved long-term survival after treatment with a specific checkpoint inhibitor,” Zebley said. “We found ASXL1 mutated in the T cells of all of these patients and decided to investigate further.”
The researchers then deleted Asxl1 in mouse T cells. They found that when blocking the checkpoints, the immune systems of these mice were better able to control tumors, and for longer, than those of mice with intact Asxl1. Further investigation revealed that deletion of Asxl1 improved treatment by preserving a small group of T cells that avoided exhaustion and maintained their anticancer effect for a year.
“We showed that disruption of Asxl1 confers superior long-term therapeutic potential to T cells, which could be a promising strategy for designing future T cell-based immunotherapies,” Zebley said.
More information:
Tae Gun Kang et al, Epigenetic regulators of clonal hematopoiesis control CD8 T cell stemness during immunotherapy, Science (2024). DOI: 10.1126/science.adl4492
Provided by St. Jude Children’s Research Hospital
Quote: Disruption of Asxl1 gene prevents T cell exhaustion to improve immunotherapy, researchers find (October 10, 2024) retrieved October 10, 2024 from
This document is subject to copyright. Except for fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for informational purposes only.