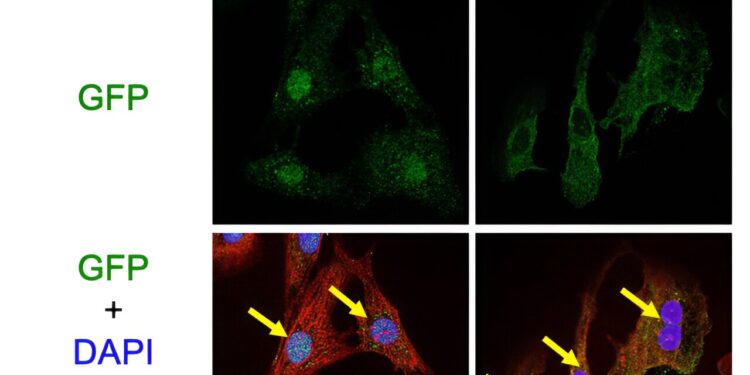
Mst1 induces the transport of FoxO1 from the cell cytoplasm to the nucleus by phosphorylating it. This is demonstrated by the colocalization of green and blue staining when Ad-Mst1 is added. Credit: Department of Cardiovascular Medicine, TMDU
Understanding the mechanisms underlying cell death and survival is critical in diseases such as heart failure, which affects millions of people worldwide. Japanese researchers have identified a mechanism that protects cardiac myocytes from ischemia, or lack of blood supply.
In their study published in Nature CommunicationsResearchers at Tokyo Medical and Dental University (TMDU) in Japan have identified a cell signaling pathway that stimulates protective mechanisms in cardiac myocytes, potentially opening avenues for the development of new therapies.
The FoxO (Forkhead box O) family of proteins is involved in many cellular functions and their cellular activity is tightly controlled. Dr. Maejima Yasuhiro, author of the study, explains: “The most puzzling aspect of the cellular function of FoxO is that it regulates both the mechanisms that promote and inhibit cell death, even in the same cells.”
The TMDU researchers therefore focused on the role of FoxOs as well as mammalian sterile kinase 1 (Mst1), which is known to interact with FoxOs to regulate processes such as cell survival. They found that Mst1 binds to and phosphorylates FoxO1.
Additionally, when Mst1 and FoxO1 were expressed together in cardiac myocytes, it increased the activity of genes that produce protective antioxidants, while suppressing genes involved in cell death.
But how does this protective mechanism work? To answer this question, the researchers took a closer look at the genes targeted by FoxO1. They found that antioxidant genes had binding sites for both FoxO1 and another protein called C/EBP-β, while genes involved in cell death had binding sites only for FoxO1.
Subsequently, further experiments showed that in the presence of FoxO1, Mst1 phosphorylated C/EBP-β. This increased FoxO1-C/EBP-β binding, which then stimulated antioxidant production and other survival mechanisms.
What effect does this mechanism have on heart cells? In mice genetically engineered to lack FoxO1 or C/EBP-β in their hearts, exposure to ischemia for four hours actually results in an increase in the amount of dead heart tissue.
In contrast, when mice lacking FoxO1 were engineered to express a phosphorylated form of C/EBP-β, the amount of dead tissue in the heart was decreased. Taken together, these results showed that this Mst1-FoxO1-C/EBP-β interaction protected the heart against ischemia.
In the long term, these results could pave the way for the development of new treatments for heart failure. “If the phosphorylation level of C/EBP-β can be increased without activating Mst1, it may be possible to promote cell survival without activating the harmful functions of Mst1,” explains Professor Junichi Sadoshima.
In other words, drugs that can selectively promote the protective functions of Mst1 would help protect cardiac myocytes in life-threatening diseases such as heart failure.
This study not only improves our understanding of the mechanisms that govern cell death and survival, but also brings new hope to patients suffering from heart failure.
More information:
Yasuhiro Maejima et al, Mst1-mediated phosphorylation of FoxO1 and C/EBP-β stimulates cellular protection mechanisms in cardiomyocytes, Nature Communications (2024). DOI: 10.1038/s41467-024-50393-y
Provided by Tokyo Medical and Dental University
Quote:New avenues for the treatment of heart failure: Discovery of a protective mechanism in cardiac myocytes (2024, September 5) retrieved September 5, 2024 from
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without written permission. The content is provided for informational purposes only.