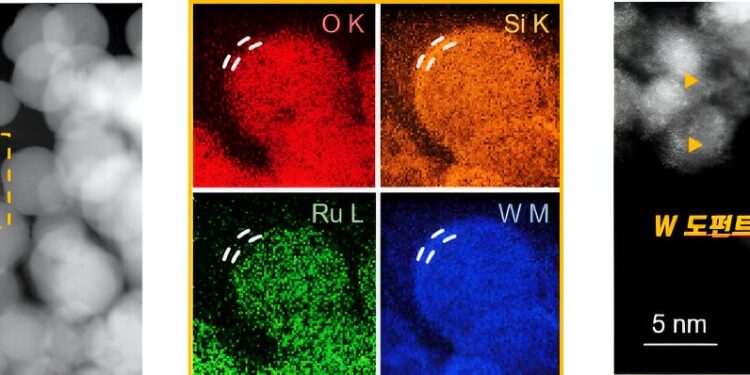
Schematic image showing the characteristics of RuSiW. (Left) Dark-field STEM, (center) EDX elemental mapping images of RuSiW, and (right) high-resolution bright-field TEM image of RuSiW. Credit: UNIST
A revolutionary technology has been developed that enables the production of green hydrogen in a more cost-effective and environmentally friendly way, bringing us closer to a carbon-neutral society by replacing costly precious metal catalysts.
Led by Professor Jungki Ryu from the School of Energy and Chemical Engineering at UNIST and Professor Dong-Hwa Seo from the Department of Materials Science and Engineering at KAIST, a joint research team successfully developed a Bifunctional water electrolysis catalyst for high efficiency and stable production of high purity green hydrogen.
The newly developed catalyst exhibits exceptional durability, even in highly corrosive acidic environments. By using ruthenium, silicon and tungsten (RuSiW), the catalyst is more cost-effective than conventional platinum (Pt) or iridium (Ir) catalysts. In addition, it emits much less greenhouse gases, making it an ecological alternative.
The study is published in the journal Advanced materials.
Water electrolysis is an advanced technology that produces hydrogen through the process of electrolysis of water. It is considered a key technology for achieving a carbon-neutral society, as it enables the production of environmentally friendly hydrogen with no carbon emissions.
The research team focused on finding alternatives to precious metal catalysts like platinum and iridium, which exhibit stability in acidic conditions. Ruthenium has attracted attention as an environmentally friendly metal due to its relatively low production cost and significantly lower greenhouse gas emissions than platinum and iridium. However, its commercialization has faced challenges due to its lower catalytic activity compared to platinum and lower stability than iridium.
Global water splitting using RuSiW as a bifunctional HER and OER catalyst. (a) Experimental design. (b) Full cell polarization curves: RuSiW on CFP as anode, RuSiW on Ti mesh as cathode and 0.5 mH2SO4 as an electrolyte. (c) Chronopotentiograms of the corresponding full cells at 10 mA cm-2. Credit: UNIST
To overcome these limitations, the research team developed a catalyst based on ruthenium, silicon and tungsten. By improving the function of the ruthenium catalyst, which exhibits lower stability in the hydrogen evolution reaction (HER) and oxygen evolution reaction (OER), the team demonstrated the catalyst’s potential as a catalyst bifunctional.
The developed catalyst has a structure doped with tungsten and silicon around a ruthenium atom. The reaction acceleration ability of the catalyst was improved by increasing the proton adsorption intensity on the catalyst surface. It exhibits higher activity in hydrogen evolution reaction compared to commercially available platinum catalysts. In addition, a thin tungsten film with a thickness of 5–10 nm protects the ruthenium catalytic site, thereby improving its stability.
Long-term stability of the corresponding catalysts during RELs at 10 mA cm-2. Credit: UNIST
The research team conducted a stability experiment on the catalyst. Using an acidic electrolyte (with an acidity of 0.3), they injected 10 mA of current into a 1 cm current.2 electrode. The developed catalyst demonstrated stable performance even after operating for more than 100 hours.
Professor Ryu said: “The development of this three-element catalyst is important because it has the potential to simultaneously replace expensive platinum and iridium. It is expected to be applied to high-purity green hydrogen production systems, such as PEM electrolyzers, because it can be synthesized easily and stably even under highly corrosive acidic conditions.
More information:
Dasom Jeon et al, Electrochemical evolution of Ru-based polyoxometalates to Si-codoped RuOx, W for the global splitting of acidic water, Advanced materials (2023). DOI: 10.1002/adma.202304468
Provided by Ulsan National Institute of Science and Technology
Quote: Discovery enables profitable and ecological green hydrogen production (January 16, 2024) retrieved January 16, 2024 from
This document is subject to copyright. Except for fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.