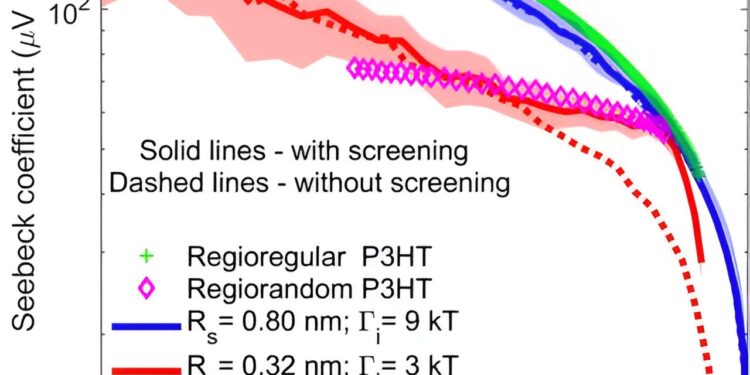
Seebeck coefficient versus electrical conductivity measurements of iodine-doped RR and RRa-P3HT measured during dedoping. We took Rd = 0.21 nm to match the radius of the iodide anion (RI = 0.206 nm). The dotted lines correspond to the case without filtering, while the bands around the simulation results represent the variance resulting from random sampling of energy sites. The jump attempt frequency is 34 for RR and 10 THz for RRa P3HT.
Silicon Valley in California and the Silicon Slopes in Utah are named for the thing most associated with semiconductors, the backbone of the computing revolution. Everything computer or electronic depends on semiconductors, a substance with properties that conduct electric current under certain conditions. Traditional semiconductors are made from inorganic materials, such as silicon, which require large amounts of water and energy to produce.
For years, scientists have been trying to create environmentally friendly alternatives using organic materials, such as polymers. Polymers are formed by linking small molecules together to form long chains. The polymerization process avoids many energy-intensive steps required in traditional semiconductor manufacturing and uses significantly less water and fewer gases and chemicals.
They are also inexpensive to manufacture and would enable flexible electronics, wearable sensors and biocompatible devices that could be introduced inside the body. The problem is that their conductivity, while good, is not as high as that of their inorganic counterparts.
All electronic materials require doping, a method of infusing molecules into semiconductors to increase conductivity. Scientists use molecules called dopants to define the conductive parts of electrical circuits. Doping organic materials has vexed scientists because of a lack of consistency: Sometimes dopants improve conductivity while other times they make it worse.
In a new study, researchers from the University of Utah and the University of Massachusetts Amherst have uncovered the physics that determines the interactions between dopants and polymers and explains the problem of incoherent conductivity.
The team found that the positively charged carriers extracted negatively charged dopants from the polymer chains, preventing the flow of electric current and reducing the conductivity of the material. However, their experiments revealed that when enough dopants were injected into the system, the behavior of the electrons changed to act as a collective screen against the forces of attraction, allowing the rest of the electrons to flow unhindered.
“The ideal case would be to inject a bunch of free electrons into the material to do the conduction work. Of course we can’t, we have to use molecules to provide the electrons,” said Zlatan Akšamija, associate professor of materials. science and engineering at the University and lead author of the study. “Our next step is to find the dopant/organic material combinations that can weaken this interaction and make the conductivity even higher. But we haven’t understood this interaction well enough to be able to deal with it so far.”
The study is published in the journal Physical Examination Letters.
Conductivity of doping juices
Electricity is a flow of electrons. Silicon alone is a poor conductor: four electrons in the outer orbital form perfect covalent bonds with nearby silicon atoms, leaving no free electrons. This is where doping comes in. Adding an impurity to silicon can do one of two things: bring additional electrons to the system or reduce the number of electrons in the system, creating positively charged carriers called holes .
For example, arsenic is a common dopant because it has five electrons in its outer orbital: four will bind to silicon and the fifth will remain free. Eventually, the dopants will provide enough free electrons to allow an electric current to flow through the silicon.
Unlike silicon, organic materials have a disordered structure in their polymer chains, leading to complicated interactions between the extra electrons from the dopant and the polymerized material, Akšamija explained.
“Imagine polymers are a bowl of spaghetti. They don’t really stack perfectly. Because of this, electrons are forced to jump from one part of the polymer to another and onto the next chain, pushed by voltage “, did he declare.
Each dopant contributes one electron at a time to the system, which means that, initially, the electrons passing through the polymer are diluted. If an electron jumps along the chain and passes near a dopant, the opposite charges will attract and cause the electron to veer off course and disrupt the electric current.
The revelation of this study was to discover that this behavior changed with a critical mass of electrons in the system: when a threshold is crossed, the crowd of electrons responds collectively. When a group of electrons passes a dopant, some are attracted to the charge and create a screen that prevents the rest of the electrons from feeling the interaction.
“And this is where screening actually does the work of blocking dopants. Carriers filter out dopants, which allows other carriers to move more efficiently. This paper describes the physical mechanism by which this happens,” he said. Aksamija said.
Experimentation and theory
Chemists from UMass Amherst conducted the physical experiments. They used two types of polymers whose structures were more or less disordered. They then used a solvent and applied it to a thin layer of glass. They then doped the polymer with iodine vapor. One of the advantages of iodine is that it is unstable: over time, the polymer gradually loses its doping molecules through evaporation.
“This was useful for the experiments because we can continue to measure the conductivity of the polymer over a period of 24 or 48 hours. This protocol gives us a conductivity curve as a function of the number of dopants remaining in the material,” said Dhandapani Venkataraman. , professor of chemistry at UMASS Amherst and co-author of the study.
“It’s an interesting trick to access almost four orders of magnitude of charge in conductivity, from low, medium and high concentrations of dopants… all the way back to essentially its original insulating state.”
The chemists conducted experiments on two different versions of the same polymer: one more regular and the other more disordered. They were then able to compare the conductivity of the two polymers as the dopant concentration changed.
“At first we were intrigued by some experimental results, especially when we had a large number of dopants. We expected that the disordered polymer would be much lower than the ordered polymer at all dopant concentrations. But this was not the case. not the case,” Venkataraman said.
Akšamija’s research group focused on the interactions of materials. They were able to compare different instances of the same polymer exhibiting greater or lesser disorders to discern where screening was occurring.
This screening behavior had never been considered part of organic semiconductor systems, so they got out some paper and pencils to understand how molecules and charges interact using first principles of physics: what is the underlying equation that governs the interaction of charges? Akšamija’s laboratory started there and rebuilt it. They then translated the formulas into code simulating electron hopping in the presence of dopants while including filtering behavior.
“We finally converged to the point where computer simulations can actually capture the experiments, not just qualitatively, but really quantitatively. The only way to align the simulation and the experiments was to include this screening effect,” Aksamija said .
Currently, the authors are using artificial intelligence to discover new combinations of polymers and dopants that can achieve the highest conductivity.
More information:
Muhamed Duhandžić et al, Carrier Screening Controls Transport in Conjugated Polymers at High Doping Concentrations, Physical Examination Letters (2023). DOI: 10.1103/PhysRevLett.131.248101. OnarXiv : DOI: 10.48550/arxiv.2311.03726
Provided by University of Utah
Quote: Uncovering the Physics of How Electrons Shield Conductivity Killers in Organic Semiconductors (February 15, 2024) retrieved February 15, 2024 from
This document is subject to copyright. Apart from fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.