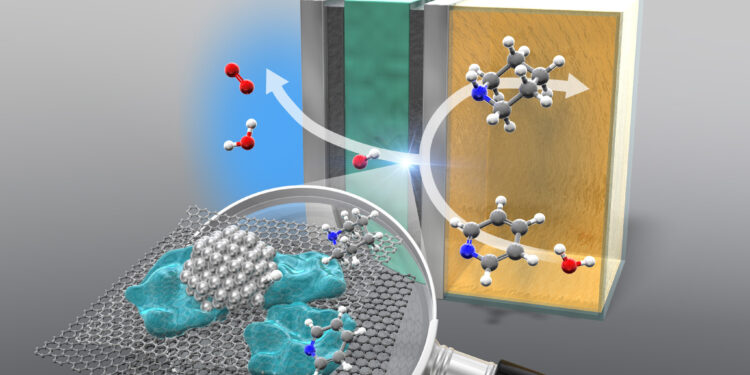
The anion exchange membrane reactor hydrogenates pyridine to piperidine. Credit: Yokohama National University
To successfully reduce the environmental impact of the chemical industry, we must find a more ecological way of manufacturing the basic chemical elements of common and massively consumed compounds.
It’s no secret that manufacturing processes have some of the largest and most intense effects on the environment, with the chemical industry leading the way in terms of energy consumption and production of emissions. While this makes sense due to the extent to which manufactured chemicals are involved in daily life, it still leaves a lot to be desired in the interest of sustainability.
By focusing on renewable energy sources and alternative methods for creating the basic chemical building blocks for some of the most commonly used compounds, researchers hope to reduce the chemical industry’s footprint through green innovations.
The researchers published their results in the Journal of the American Chemical Society on October 7.
This study mainly focuses on cyclic amines, as they are the most important building blocks in fine chemicals. These compounds are arranged in a ring and, in this case, have a nitrogen atom. One of the stars of the show is pyridine, which gives way to piperidine, a cyclic amine essential in the fine chemicals industry.
Piperidine, for example, provides the framework for many materials such as FDA-approved medications, pesticides, and everyday materials used in many people’s lives.
Typical methods for adding hydrogen to a nitrogen-containing cyclic amine involve using hydrogen gas as a source of protons and electrons. The hydrogenation process relies on hydrogen obtained by steam reforming methane, a major greenhouse gas.
Not only is this method energy-intensive, but it is also responsible for around 3% of global carbon dioxide emissions. This process is also heavily dependent on fossil fuels and consumes a large amount of energy. Fortunately, researchers have found a way around this problem by developing an anion exchange membrane (AEM) electrolyzer.
An AEM electrolyzer allows the hydrogenation of different types of pyridines at ambient temperature and pressure, without having to use acid additives as in traditional methods. The function of the electrolyzer is to divide water into its components, atomic hydrogen and oxygen. The atomic hydrogen obtained is then added to the cyclic compound.
The AEM electrolyzer also demonstrates great versatility with other nitrogen-containing aromatics, making it a promising route for a wide range of applications. Additionally, by developing a method that can be used at ambient temperatures and pressures, the electrical energy required for the process is significantly reduced.
“The method offers significant potential for industrial-scale applications in pharmaceuticals and fine chemicals, contributing to the reduction of carbon emissions and the advancement of sustainable chemistry,” said first author Naoki Shida. of the study and researcher at Yokohama National University.
This process uses water and renewable electricity as an energy source, which contrasts with the reliance on fossil fuels for the conventional method. Efficiency has not been compromised by this method and the large-scale yield percentage is 78%, further affirming that this technology can be reasonably scalable.
One problem that could be encountered is an increase in cell voltage during the electrolysis process, but this can be mitigated either by an improved AEM or, preferably, by designing an AEM specifically designed for the organic electrosynthesis.
For electrocatalytic hydrogenation technology to spread and make a difference, it must be scalable on an industrial scale so that pharmaceutical and fine chemical companies can use it. The more this technology is used, the easier it is to transition to other nitrogen-containing aromatic compounds, thus expressing the practicality of the electrocatalytic hydrogenation process.
Ideally, this method would emerge as an alternative to traditional methods used in the chemical industry and ultimately reduce the overall carbon footprint left by chemical manufacturing.
The Japan Synchrotron Radiation Research Institute made this research possible.
More information:
Electrocatalytic hydrogenation of pyridines and other nitrogen-containing aromatic compounds, Journal of the American Chemical Society (2024). DOI: 10.1021/jacs.4c09107
Provided by Yokohama National University
Quote: Efficient way to hydrogenate nitrogen-containing aromatic compounds developed (October 7, 2024) retrieved October 8, 2024 from
This document is subject to copyright. Except for fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for informational purposes only.