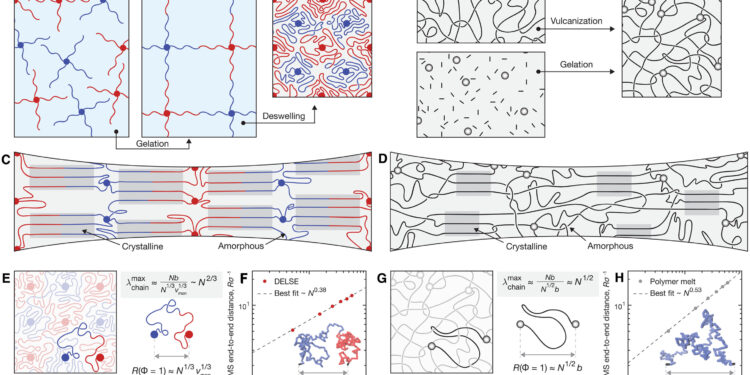
Deflated star elastomers at the ends. (A) DELSE forms by controlled cross-linking of star macromers followed by solvent evaporation to form a homogeneous cross-linked polymer network (illustrations are exaggerated to highlight architectural differences). (B) Conventional elastomers form through random cross-linking processes such as vulcanization of long polymer chains or gelation from monomers. (C) The more homogeneous architecture supports chain alignment during stretching, causing the formation of crystalline domains in DELSE. (D) In contrast, physical barriers such as trapped entanglements and inhomogeneities limit the effect of SIC in common elastomers. (E) The end-to-end RMS distance of polymer chains in a DELSE in the undeformed state scales as N1/3, as validated by (F) molecular dynamics simulation (representative simulated chain conformation inset ). (G) The end-to-end RMS distance of polymer chains in a conventional elastomer in the undeformed state scales as N1/2, as validated by (H) molecular dynamics simulation (simulated chain conformation inset representative). Credit: Scientists progress (2023). DOI: 10.1126/sciadv.adj0411
Strain-induced crystallization can strengthen, harden, and facilitate an elastocaloric effect in elastomers. The resulting crystallinity can be induced by mechanical stretching in common elastomers which is generally less than 20%, with a plateau in extensibility.
In a new report now published in Scientists progressChase M. Hartquist and a team of mechanical engineering and materials science scientists from MIT and Duke University in the United States used a class of end-bonded elastomers to achieve strain-induced percentage crystallinity .
The deflated and end-bonded star elastomer, abbreviated as DELSE, achieved ultra-high scale extensibility, beyond the saturated limit of common elastomers, to promote high elastocaloric effect with adiabatic temperature change.
Stress-induced crystallization
The process of strain-induced crystallization is common in elastomers and gels, where amorphous polymer chains can transform into highly oriented and aligned domains due to applied mechanical stress. Since the oriented and aligned crystal domains can resist crack extension and blunting to facilitate crack deflection, the deformation-induced crystallization process preserved the network integrity, while achieving recovery close to 100% in a few seconds.
The method plays a key role in various applications including elastocaloric cooling and strain-based actuation.
The typical process of strain-induced crystallinity in common elastomers is less than 20%, while natural rubber only achieves about 15% crystallinity when stretched to six times its original length at room temperature . In this new work, Hartquist and a team of researchers described a class of deflated, end-bonded star elastomers that achieve up to 50% strain-induced crystallinity. The scientists attributed the ultra-high stress-induced crystallization to uniform lattice structure and high extensibility to achieve the expected results.
Ultra high SIC of DELSE. (A) WAXS and SAXS models show the structural development of DELSE at 55°C compared to DELE at 55°C and NR at 22°C when mechanically stretched. The WAXS intensity profile develops crystal peaks for (B) DELSE and (C) NR during stretching. (D) The crystallinity index increases more dramatically for DELSE than for NR. (E) Deconvolution of WAXS scans gives the distribution of oriented and unoriented phases. (F) Deformation-induced crystallinity of DELSE measured from deconvolution of the WAXS pattern is compared to DELE and values reported for various common rubbers with SIC. Error bars indicate standard deviations. Credit: Scientists progress (2023). DOI: 10.1126/sciadv.adj0411
To study additional characteristics of the elastomer, the team used X-ray analysis to show how the structure and stress-induced deflated, end-bonded star elastomer promoted crystallinity compared to common elastomers. The research team then analyzed the formed crystal structure using detailed X-ray analysis, where the deflated and end-bonded star elastomers showed a diffraction point to mark the formation of poly(doxide) crystals. ethylene) in a helical structure. This elastomer promotes higher strain-induced crystallinity than common elastomers.
Mechanical performance and elastocaloric cooling
The research team performed mechanical characterization at 60°C to study ultra-high strain-induced crystallization in end-deflated elastomers, which effectively promoted high toughness, with low strain-stretch hysteresis. Hartquist and the team strengthened the softer materials by introducing reversible bonds to induce large stress-stretch hysteresis.
The researchers then studied the stretchability of elastomers to show how the materials stretched beyond the limits of entangled networks for broader applications. They then investigated the potential of using a caloric material for solid-state cooling applications by studying the elastocaloric effect in end-deflated star elastomers, and compared the results with conventional elastomers.
Scientists investigated the possibility of using a caloric material for solid-state cooling applications by studying the elastocaloric effects of deflated star elastomers compared to natural rubber. An ideal elastocaloric cooling cycle can exploit the decrease in entropy conformation to increase thermal entropy and heat the bulk material.
In strain-induced crystallization elastomers, additional latent heat contributed to the formation of crystallites to enhance the effect. The increased extensibility and uniform chain length distribution of the material increased the theoretical elastocaloric effect, compared to conventional elastomers. These elastomers were strong candidates suitable for advanced solid-state cooling technologies.
Elastocaloric effect of DELSE. (A) Schematics indicate destruction of crystal domains and disruption of polymer chain alignment upon adiabatic retraction. (B) Thermal images of DELSE during retraction. The applied mechanical load and measured surface temperature are recorded for the (C) DELSE and (D) NR during the shrinking process. Credit: Scientists progress (2023). DOI: 10.1126/sciadv.adj0411
Outlook
In this way, materials scientists Chase M. Hartquist and colleagues compared the deflated and end-bonded star elastomer with natural rubber to show their increased stability, different polymer chemistry, and well-formed structure that increased combinatorially. strain-induced crystallization and the elastocaloric effect in elastomeric materials. Comparison between the materials revealed their extensibility and chemistry, as well as the importance of their relatively homogeneous structure.
Since the discovery of elastic by JR Katz in 1924 through stress-induced crystallization, this biomaterial has played an important role in society, from household items to car tires. In this report, the team described next-generation elastomers developed with stress-induced deep crystallization that exceeded the dimensions of natural rubber and other common materials.
The developed materials showed the ability to outperform their conventional counterparts, suggesting the ability to engineer soft materials by regulating their network architecture. These materials play a crucial role in building futuristic aerospace structures, medical devices and for elastocaloric refrigeration applications.
More information:
Chase M. Hartquist et al, An Ultra High Strain Induced Crystallization Elastomer, Scientists progress (2023). DOI: 10.1126/sciadv.adj0411
© 2023 Science X Network
Quote: Development of a futuristic elastomer with ultra-high strain induced crystallization (December 27, 2023) retrieved December 27, 2023 from
This document is subject to copyright. Apart from fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.