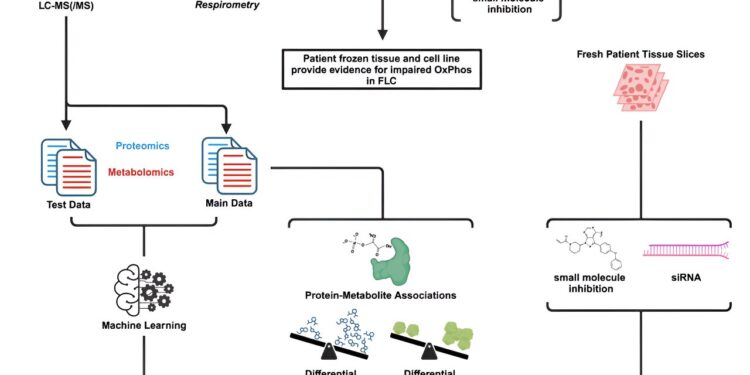
Graphic summary. Credit: Reports on medicine cells (2024). DOI: 10.1016/j.xcrm.2024.101699
A deadly liver cancer that primarily affects children and young adults reconfigures its cellular metabolism to thrive, according to a new study that opens the door to exploring new therapeutic targets.
Fibrolamellar carcinoma, which does not respond to conventional treatments, accounts for only 1 to 2% of all liver cancers, but it does not receive standard care and has often metastasized by the time it is detected, leaving patients with an average of about a year to live.
While many cancers prefer glucose to aid their survival, some are dependent on other nutrients. Fibrolamellar carcinoma relies on glutamine for energy production and also appears to place a high premium on using serine for this purpose, the study found.
“There appears to be an alteration in sugar metabolism with this cancer and it actually appears to be very fond of amino acids, which is something that is out of the norm in terms of what the general public generally thinks about cancer,” said Donald Long Jr., the study’s senior author, a Howard Hughes Medical Institute Gilliam Fellow, and a doctoral student in the lab of senior author Praveen Sethupathy ’03, professor of physiological genomics and chair of the Department of Biomedical Sciences in the College of Veterinary Medicine.
The article, “Proteo-metabolomics and Patient Tumor Slice Experiments Point to Amino Acid Centrality for Rewired Mitochondria in Fibrolamellar Carcinoma,” published August 28 in the journal Reports on medicine cells.
“Our results highlight specific features of cancer that represent therapeutic vulnerabilities and could be very useful for testing specific drug combinations,” Sethupathy said.
In this study, the researchers used proteomics (to determine the spectrum of proteins in cancer cells) and metabolomics (the spectrum of metabolites) and identified more than 8,000 proteins and 135 metabolites. They obtained frozen tissue samples from patients from the Fibrolamellar Cancer Foundation Biobank and developed a predictive model based on the omics data, generated in collaboration with co-author Lukas Orre, associate professor at Karolinska Institutet in Sweden.
Several key aspects of the model were experimentally validated through functional studies using fresh tumor tissue slices directly from patients, in collaboration with co-author Taranjit Gujral, associate professor at the Fred Hutchinson Cancer Center.
Under normal circumstances, the glycolytic pathway breaks down glucose to form a metabolite called pyruvate, which then enters the mitochondria (the cell’s powerhouse) and is used to produce ATP, a small molecule that stores chemical energy used to power the cell’s biochemical reactions.
“We found, from our analysis of proteomic and metabolomic data and additional experiments in a fibrolamellar carcinoma cell model, that this pathway was altered,” Long said.
The researchers then identified a liver-specific protein called serine dehydratase as the tenth most upregulated protein in the proteomics dataset. This enzyme can convert the amino acid serine into pyruvate. Pyruvate then follows the usual pathway into the mitochondria to produce ATP.
In tests on fresh cancer tissue sections from patients, the researchers inhibited serine dehydratase and found that the cancer tissue became much less viable. A drug that blocks pyruvate from entering mitochondria also had a significant effect on the survival of fibrolamellar cancer tissue.
The researchers also foresee another use for pyruvate, where the metabolite would also be used in the production of proline. Proline is a non-essential amino acid that plays a critical role in making collagen, the main building block of connective tissue.
One of the characteristics of fibrolamellar carcinoma is that its tumors are crossed by fibrous bands of collagen.
“It looks like, potentially, the mitochondria are being reconfigured to produce large amounts of proline, presumably to integrate into this collagen matrix that promotes tumor growth,” Long said.
Co-authors also included Rosanna Ma and Adam Francisco of Sethupathy’s lab; Nathaniel Vacanti, assistant professor, Joeva Barrow, assistant professor, and Pei-Yin Tsai, doctoral student, both of the Division of Nutritional Sciences in the College of Human Ecology; and researchers from UCLA, Johns Hopkins University School of Medicine and Rutgers University.
More information:
Donald Long et al., Proteometabolomics and patient tumor slice experiments indicate centrality of amino acids to rewired mitochondria in fibrolamellar carcinoma, Reports on medicine cells (2024). DOI: 10.1016/j.xcrm.2024.101699
Provided by Cornell University
Quote:Deadly liver cancer reprograms cellular metabolism to thrive, study finds (2024, August 28) retrieved August 28, 2024, from
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without written permission. The content is provided for informational purposes only.