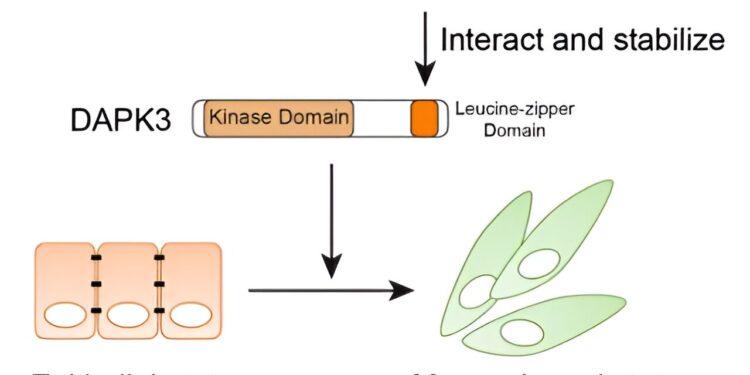
Graphical representation of the functional role of DAPK3 in TNBC cells. Credit: Nexus PNAS (2024). DOI: 10.1093/pnasnexus/pgae401
Triple negative breast cancer (TNBC) is the most difficult subtype of breast cancer to treat. TNBC patients account for more than 20,000 cases of this disease each year in the United States alone. Their outcomes are worse than those of patients with other breast cancer subtypes: their five-year mortality rate is around 40%. The high mortality rate is thought to result from the propensity of cancer cells to spread or metastasize to other organs and the lack of effective cancer-specific therapies.
At Baylor College of Medicine, Dr. Charles Foulds, assistant professor at the Lester and Sue Smith Breast Center and member of the Dan L Duncan Comprehensive Cancer Center, and his Baylor colleagues are conducting research with the goal of better understanding TNBC and potentially identify vulnerabilities that could lead to more effective therapies.
Scanning for TNBC vulnerabilities with KIPA
“In this study, published in Nexus PNAS“We looked for enzymes called kinases, whose expression is typically altered in cancer,” said the work’s first author, Dr. Junkai Wang, who was a member of the Foulds lab when working on this project.
Previous laboratory studies have shown that targeting kinases can be therapeutically effective in other cancers. There are many inhibitors of these enzymes that are already approved by the Food and Drug Administration for human use and could be tested for their potential therapeutic value in TNBC. “The challenge was to identify the kinase among hundreds of kinases in TNBC cells that could potentially give us an advantage against this cancer,” Wang said.
The researchers used a laboratory method they had previously developed, called the kinase inhibitor pull-down assay (KIPA), which significantly speeds up the process of identifying kinases from hundreds of potential candidates.
Working with 16 patient-derived xenografts (PDX), human breast cancer tumors grown in immunocompromised mouse models, the team used KIPA to look for kinases that were significantly altered in quantity in TNBC, compared to cells. normal.
“We found that TNBC cells contain more of a kinase called Death-Associated Protein Kinase 3 (DAPK3),” Wang said. “We confirmed this finding in TBNC cell lines and in tumors.”
Importantly, while protein levels of DAPK3 were higher than normal in TNBC, levels of its precursor mRNA were not. The mRNA molecule carries the genetic sequence of the DAPK3 gene and is used by the cell to synthesize the protein.
“Many studies rely solely on mRNA data to assess which proteins are produced by cells,” Wang explained. “If we had just looked at mRNA and not protein levels in TNBC, we would not have realized that the DAPK3 protein is overproduced in this cancer and deserves further attention.”
A new role for DAPK3
Other studies showed that removing the DAPK3 protein by deleting the DAPK3 gene did not affect the growth of cancer cells; however, it prevented the migration and invasion of TNBC cells in laboratory experiments. When TNBC cells with the inactivated DAPK3 gene were cultured as tumors in immunocompromised mice, no significant effect on tumor metastasis was observed. Further modeling of metastases is necessary to provide a definitive result.
Researchers also gained new insights into how DAPK3 exerts its migration-promoting effects.
“We found that DAPK3 reduces the levels of desmoplakin (DSP), a protein involved in the regulation of cell adhesion, linked to a cell’s ability to migrate,” Wang said. “In addition, we discovered that a protein called LUZP1 binds to DAPK3, which protects it from destruction by the cell.”
“Overall, our results have improved our understanding of the kinases that control TNBC cell spreading,” Foulds said. “We previously thought that a cancer driver might affect cell proliferation and migration. But we found that in TNBC, DAPK3 does not regulate growth, but controls migration and invasion. Our next steps include making Additional studies to learn more about how DAPK3/LUZP1 complex functions promote TNBC migration and evaluate its potential value as a therapeutic target.
Other contributors to this work include Anh M. Tran-Huynh, Beom-Jun Kim, Doug W. Chan, Matthew V. Holt, Diana Fandino, Xin Yu, Xiaoli Qi, Jin Wang, Weijie Zhang, Yi-Hsuan Wu, Meenakshi Anurag. , Xiang HF Zhang, Bing Zhang, Chonghui Cheng, and Matthew J. Ellis. The authors are affiliated with Baylor College of Medicine.
More information:
Junkai Wang et al, Death-associated protein kinase 3 modulates migration and invasion of triple-negative breast cancer cells, Nexus PNAS (2024). DOI: 10.1093/pnasnexus/pgae401
Provided by Baylor College of Medicine
Quote: DAPK3 appears as a new regulator of triple negative breast cancer cell migration (October 8, 2024) retrieved October 8, 2024 from
This document is subject to copyright. Except for fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for informational purposes only.