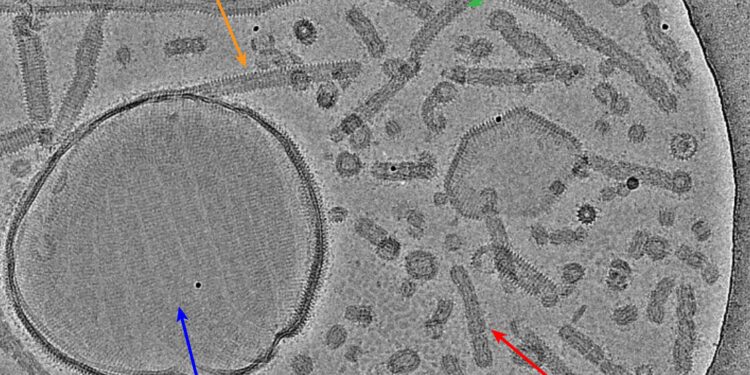
Cryo-EM shows a multitude of different Vipp1 structures: carpet-like structures, ring assemblies, and tubes. Credit: Forschungszentrum Jülich / Bendikt Junglas, Carsten Sachse
The membrane that surrounds the cells of living organisms is extremely flexible and sensitive. How it protects itself from damage and renews itself is crucial for many life processes and is not yet fully understood in detail. Scientists at Forschungszentrum Jülich have now been able to gain fascinating new insights using electron cryomicroscopy.
Their work is published in the journal Nature Structural and molecular biology.
The membrane protein Vipp1, known from the photosynthetic apparatus of plants, algae and bacteria, can form various structures that could serve as tools to stabilize the cell membrane and strengthen it if necessary.
In a second study published in the same journal, the researchers were also able to gain new insights into the function of the related protein PspA, present in bacteria. The two molecules, Vipp1 and PspA, are unusually plastic and can adopt different structures, creating rings and tubes of different diameters.
The cell membrane performs many important functions. For example, it protects the interior of the cell from the environment. At the same time, nutrients are absorbed across the cell membrane, wastes are excreted, and signals are transmitted between cells.
Despite its central role, the cell membrane is also very sensitive. It is a thin layer of lipids which, although protective in themselves, are also sensitive to stress caused by physical pressure, stretching or chemical influences. Environmental factors such as UV rays or toxins can also damage the membrane.
In plant cells, for example, intense light can severely stress or even damage the membranes of chloroplasts, where photosynthesis takes place. Proteins like Vipp1 are therefore essential for cell survival, because they protect membrane structures and repair them if necessary.
Exactly how the mechanism works is not yet fully understood. However, thanks to state-of-the-art cryoelectron microscopes, researchers have now been able to gain new insights into the interaction between Vipp1 and the cell membrane. They found that Vipp1 forms carpet-like structures on the cell membrane and stabilizes it. Furthermore, they found ring complexes and tubes made of membrane-filled Vipp1, which can possibly “pin off” damaged membrane areas as well as connect two separate membranes.
These findings provide new insights into the ability of Vipp1 and PspA proteins to modify cell membranes and thus protect vital cell processes. These discoveries could contribute to the development of new biotechnological applications in the future, such as the production of biomaterials or the optimization of photosynthesis in plants.
Vipp1 is particularly important because it is involved in the formation and maintenance of thylakoid membranes, the chloroplast membranes of plant cells where the light reaction of photosynthesis takes place, that is, the conversion of light into chemical energy. .
Interestingly, the basic mechanism is very similar to that of ESCRT-III proteins, which are also highly conserved in human cells. These proteins have remained essentially unchanged during evolution, indicating an important function. A better understanding of the structure and function of these proteins could thus lead to the development of new drugs, such as antibiotics, targeting cell membrane processes.
Cryoelectron microscopes from the Ernst Ruska Center (ER-C) at the Forschungszentrum Jülich were used in both studies. Microscopes have allowed researchers to study proteins at atomic resolution and observe them in an unusually high number of structural states as well as interactions between proteins and membranes. These studies are part of an established collaboration with the research group of Professor Dr. Dirk Schneider at the Johannes Gutenberg University Mainz.
More information:
Benedikt Junglas et al, Structural basis of Vipp1 membrane binding: from loose coats and mats to ring and rod assemblies, Nature Structural and molecular biology (2024). DOI: 10.1038/s41594-024-01399-z
Benedikt Junglas et al, Structural plasticity of the bacterial ESCRT-III protein PspA in higher order assemblies, Nature Structural and molecular biology (2024). DOI: 10.1038/s41594-024-01359-7
Provided by Forschungszentrum Juelich
Quote: Cryoelectron microscopy provides new insights into the cellular repair system (October 8, 2024) retrieved October 8, 2024 from
This document is subject to copyright. Except for fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for informational purposes only.