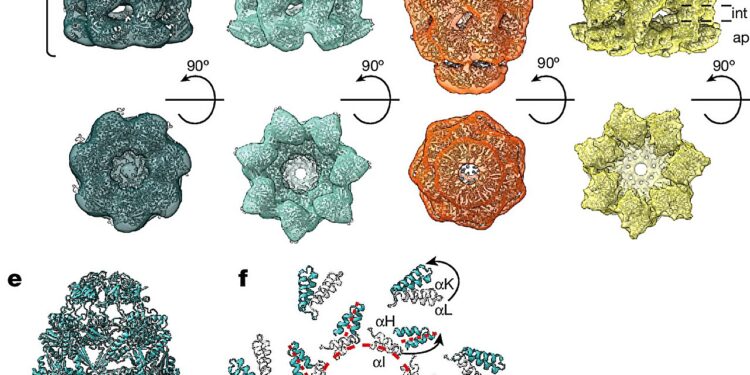
In situ structures of chaperonin complexes. Credit: Nature (2024). DOI: 10.1038/s41586-024-07843-w
Cryo-electron tomography (cryo-ET) makes it possible to visualize and analyze cellular structures in their natural environment. Researchers from the MPI for Biochemistry Martinsried and the University Medical Center Göttingen used cryo-ET to study protein folding helpers, called chaperonin complexes, in the bacterium E. coli.
These chaperonins help newly synthesized proteins fold into their correct functional shape. The researchers were able to shed light on the folding reaction in unprecedented detail, monitoring the conformational changes of the chaperonin as well as its interactions with the client protein inside the folding chambers.
The results were published in Nature.
Protein folding auxiliary chaperonin
Proteins are the basic molecular building blocks of life. To perform a multitude of functions in the cell, each protein must adopt a defined three-dimensional structure, similar to the components of machines. Protein folding assistants, called chaperonins, help newly synthesized proteins achieve their functional form.
Biochemist F.-Ulrich Hartl, Director of the MPI for Biochemistry, explains: “I have been working with chaperonins for over 30 years. This protein complex exists unchanged in almost all living organisms and is essential for the correct folding of proteins and the survival of cells. Misfolded proteins are associated with diseases such as Alzheimer’s and Parkinson’s. Understanding the structure and function of chaperonins could help us develop new strategies for the treatment of these diseases.”
To better understand how chaperonins work, Hartl collaborated with structural biologists Wolfgang Baumeister, Emeritus Director and inventor of cryo-ET at the MPIB, and Rubén Fernández-Busnadiego of the Institute of Neuropathology at the University Medical Center Göttingen (UMG) and member of the Cluster of Excellence “Multiscale Bioimaging: From Molecular Machines to Excitable Cell Networks” (MBExC).
Chaperonin complex
In bacteria, chaperonin complexes consist of two distinct subunits, GroEL and GroES. GroEL is organized into two stacked rings that form a barrel. GroES acts as a lid for the GroEL barrel. Newly produced proteins are encapsulated in the nano-sized barrel and can fold while being protected from the cellular environment.
The researchers were able to identify two main forms of the GroEL-GroES complex in cells, called “ball” and “football” (after the shape of an American football). These forms differ in their structural symmetry. In the ball form, a GroES capsule is attached to only one side of the GroEL body. This form was found mainly in normally growing bacteria. Football-shaped complexes were also detected.
The microscopic images also showed that the proteins to be folded were located in the chaperonin barrel. Jonathan Wagner, a researcher at Martinsried and Göttingen, explains: “It is fascinating that cryo-electron microscopy is now so advanced that we can follow processes such as protein folding in such detail in living cells.”
Fernández-Busnadiego explains: “In this study, we combined cryo-ET with single-particle cryo-electron microscopy (cryo-EM) and quantitative mass spectrometry. This allowed us to observe different conformations of chaperonin complexes in different cellular states and determine their abundance. The ability to visualize these complexes directly in the bacteria instead of just in the test tube represents a major advance in the field and has only recently become possible, as this chaperonin complex is only 14 nanometers wide.”
“Decades of experiments with purified GroEL/ES complexes have led to conflicting results on how this mechanism works. This is likely because in vitro experiments cannot fully reproduce the conditions found inside the cell. We can now solve this conundrum using cellular cryo-ET, as we can image the complexes at high resolution in native, unperturbed cellular environments.”
Hartl concludes: “The results indicate that during chaperonin-mediated protein folding, proteins assemble differently and alternate between the asymmetric “ball” shape and the symmetric “football” shape during the same reaction cycle. In our future work, we will focus on elucidating the intermediate states of these cycles to understand how they are regulated by the chemical reactions of ATP binding and hydrolysis.”
More information:
Ulrich Hartl, Visualization of chaperonin function in situ by cryo-electron tomography, Nature (2024). DOI: 10.1038/s41586-024-07843-w. www.nature.com/articles/s41586-024-07843-w
Provided by the Max Planck Institute for Biochemistry
Quote: Cryo-ET study elucidates protein folding helpers in their natural environment (2024, August 21) retrieved August 21, 2024 from
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without written permission. The content is provided for informational purposes only.