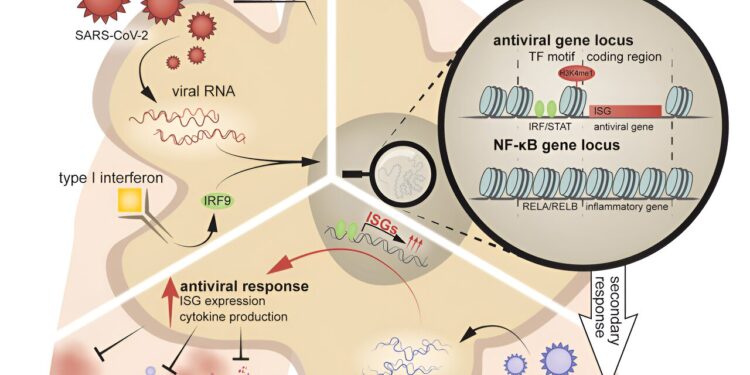
Graphical summary. Credit: Immunity (2024). DOI: 10.1016/j.immuni.2024.08.018
More than 200 viruses can infect and cause disease in humans; Most of us will be infected with several diseases during our lifetime. Does an encounter with one virus influence how your immune system responds to another? If yes, how? Does it weaken your defenses, strengthen them, or have some other impact?
These are questions facing scientists at Rockefeller University’s Laboratory of Virology and Infectious Diseases, led by Charles M. Rice, and Weill Cornell Medicine’s Laboratory of Epigenetics and Immunity, led by Steven Z. Josefowicz, teamed up to answer in a new study published in the journal Immunity.
By analyzing mice that had been infected first with SARS-CoV-2 and then with the influenza A virus, scientists found that having recovered from COVID had a protective effect against the worst effects of the flu, and that this memory response came from an unexpected corner of the immune system.
It turned out that epigenetic changes in macrophages – innate immune cells that are among the first to respond to a threat – had developed a kind of “memory” following COVID that allowed these cells to better defend themselves against an unrelated virus. It was long thought that immunological memory was limited to adaptive immune cells, although recent work has called this dogma into question. Even more intriguing, what the macrophages remembered was not specific to any particular virus.
The findings improve our understanding of innate immune memory and could allow researchers to exploit the phenomenon in new ways to create therapies that confer widespread protection against multiple viruses.
“Immune memory is essential for combating recurrent diseases caused by pathogens. What is exciting about our study is that we discovered broadly effective antiviral immune memory in macrophages following SARS infection- CoV-2, which can reduce disease caused by a completely different virus,” says first author Alexander Lercher, a postdoctoral researcher in the lab.
“A more detailed understanding of these mechanisms could facilitate the development of new therapeutic strategies covering a range of respiratory viruses,” says Rice.
“It was so exciting to team up with Alex and Charlie and delve into the epigenetic mechanisms coding for this general antiviral memory,” adds Josefowicz. “The implications are profound. If we can walk around with boosted immunity for months after a season of respiratory infections, what are the implications for seasonal trends in these infections? To what extent does human variance – genetic and epigenetic – exist is she in these ways?
Waterfall effect
When a virus invades the body, signaling molecules called cytokines tell innate immune cells like macrophages to pursue and consume whatever sounds their alarm. This universal approach is followed by a targeted attack of adaptive immune cells such as T cells, which identify a specific antigen of the virus, adapt their attack towards it and remember it in the long term to fight against future invasions of the virus . same virus.
However, findings over the past two decades show that innate immune responses can lead to cellular memory. In several studies, for example, researchers found that people who received the live attenuated Bacillus Calmette-Guérin vaccine, which aims to protect against tuberculosis, elicited memory innate immune responses that lasted for months and provided protection against unrelated infections.
But how this broadly effective immune memory develops is little understood. In 2020, Lercher began to study the phenomenon using widely circulating viruses: SARS-CoV-2, then the most dominant global pathogen, and the influenza A virus, a recurring scourge that has struck humanity since the pandemic. of 1918, when it jumped from birds to humans, spreading around the world and killing millions.
Flip the switch on genes
Lercher and colleagues set out to study the long-term consequences of past SARS-CoV-2 infection on the respiratory system. They focused their analysis on cells in the lungs and discovered that alveolar macrophages, located in the airways, acquired a new epigenetic program after infection. Specifically, they found that the chromatin that packages genes was more accessible around antiviral genes, making them “ready to go” after recovery from COVID.
These results are not limited to mice. When analyzing samples from people who had recovered from mild COVID, researchers found similar epigenetic changes in blood monocytes, the progenitor cells of macrophages.
The result of this epigenetic reprogramming is memory of previous infections and an altered immune response to future infections.
RAM
Because macrophages in the lungs of COVID-recovered mice had acquired antiviral innate immune memory imprinted on their chromatin, they could more successfully fight disease caused by a new viral invader. Compared to naive mice, they had fewer symptoms of influenza A, such as significant weight loss or dysregulated inflammatory responses, and lower mortality rates.
“The fact that viral RNA alone seems to be able to trigger memory in macrophages lays the foundation for the independence of this memory towards antigens,” explains Lercher. “They recognize a pattern shared by many viruses, as opposed to a virus-specific antigen.”
The researchers confirmed this by exposing mice to a synthetic mimic of an RNA virus and found memory responses similar to those observed after SARS-CoV-2 infection.
Interestingly, when it came to fighting secondary influenza infection, memory-adapted macrophages outperformed adaptive T cells. “It’s really the macrophages that drive this response,” says Lercher.
Finally, to test the memory of macrophages, the researchers extracted them from recovered mice, transferred them to naive mice, and then infected these mice with the influenza A virus. So, if the recovered macrophages were up to the task, recipient mice should develop less severe illness when infected with influenza A.
They were. “Naive mice with implanted rescued macrophages resisted influenza better than mice implanted with naive macrophages,” says Lercher.
Pandemic Preparedness
In the future, researchers want to identify which factors are critical for the establishment of innate immune memory. “In an ideal world, we would find one or a few factors leading to this memory formation in macrophages and other innate cells, and then exploit it to develop therapies that provide broad protection against many viruses,” says Rice.
This approach could be particularly useful in the face of a possible pandemic. “If there was a new emerging pathogen on the horizon, for example, it would be nice to have a treatment that would boost your general antiviral immunity for about a month,” says Lercher. “It’s still a long way off and there’s still a lot of research to be done, but I think it might be possible one day.”
More information:
Alexander Lercher et al, Antiviral innate immune memory in alveolar macrophages following SARS-CoV-2 infection ameliorates secondary influenza A virus disease, Immunity (2024). DOI: 10.1016/j.immuni.2024.08.018
Provided by Rockefeller University
Quote: COVID-induced immune memory could protect against severe cases of flu, mouse study suggests (September 30, 2024) retrieved September 30, 2024 from
This document is subject to copyright. Except for fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for informational purposes only.