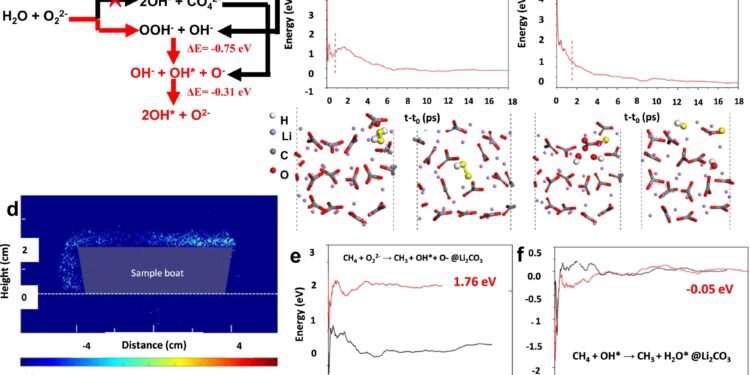
Probe of the evolution of OH radicals. A Summary of possible reaction product of H2O + O22−; b, vs: Average energies as a function of elapsed time (tt0) for the evolution of H2Oh2+ O2− and O.H.− +CO42−in melted Li2CO3, respectively. The electrophilic oxygen atoms involved in the reactions are highlighted in yellow to allow better visualization; d LIF experiments on SiO2@5Li2CO3the scale bar indicates the relative intensity of the OH radical; e And F:, respectively. Credit: Natural communications (2023). DOI: 10.1038/s41467-023-43682-5
Ethylene is sometimes considered the most important chemical in the petrochemical industry because it serves as a raw material for a wide range of everyday products. It is used to produce antifreeze, vinyl, synthetic rubber, foam insulation and plastics of all kinds.
Currently, ethylene is produced by an energy and resource-intensive process called steam cracking, where extreme temperatures and pressures produce ethylene from crude oil in the presence of steam and, in doing so, emit carbon dioxide. tons of carbon dioxide in the atmosphere.
However, another way to produce ethylene is through a process called oxidative methane coupling (OCM). It has the potential to be a greener alternative to steam cracking, but until recently the amount of ethylene it produces did not make the process economically viable.
“So far, the catalytic efficiency has been less than 30% for a single pass, which simply means passing methane and oxygen through the catalyst and getting ethylene on the other side,” explains Bar Mosevitzky Lis, postdoctoral research associate in the Department of Chemistry. and biomolecular engineering in the PC Rossin College of Engineering and Applied Science at Lehigh University.
“Studies that have simulated the entire industrial process using OCM have shown that the technology only becomes cost-effective when the single-pass yield reaches between 30 and 35 percent.”
OCM is now about to leave the laboratory and enter the real world. For the first time, researchers from North Carolina State University (NCSU) and Lehigh University, in collaboration with researchers from the Guangzhou Energy Conversion Institute and the University of Science and Technology Technologies from East China, have developed an OCM catalyst that exceeds 30% when used. comes to the production of ethylene.
The paper describing their breakthrough was recently published in Natural communications.
The collaboration was led by Fanxing Li, Alcoa professor of engineering at NCSU. His team developed a Li core-shell class2CO3-coated rare earth mixed oxides as catalysts for the oxidative coupling of methane using a chemical loop scheme. The result was a single pass yield of up to 30.6%.
“The idea of chemical looping is that instead of co-feeding methane and oxygen into the chamber with the catalyst, you do it sequentially,” explains Mosevitzky Lis, who is also one of the co-authors of the study.
“Over time, you lose oxygen from the catalyst and it becomes ineffective. With chemical looping, you start with methane, then switch to oxygen, then switch back to methane, and the oxygen is used to continually reoxidize the catalyst, thereby replenishing its ability to provide oxygen for the reaction.”
Mosevitzky Lis and his Lehigh team, led by Israel Wachs, the G. Whitney Snyder Professor of Chemical and Biomolecular Engineering and director of the Operando Molecular Spectroscopy and Catalysis Research Laboratory, characterized the catalyst.
“Our specialization is in situ surface characterization,” explains Mosevitzky Lis, “which means we characterize the surface of catalysts while the reaction is in progress. We apply a wide range of physical and chemical techniques to understand the transformations that catalysts undergo during the course of the catalytic reaction. on their surface and how these transformations are linked to what makes them such good catalysts.
He says the catalyst is composed of a mixed oxide core coated with lithium carbonate and the interaction between the core and shell during chemical looping is responsible for the high efficiency. These results mean that, for the first time, transforming methane, found in natural gas and biogas, into ethylene could be within reach of industry.
“OCM has the potential to be cheaper and more efficient in terms of energy and emissions,” he says. “Also, instead of using crude oil, you use methane which usually comes from natural gas, but could also be generated in the future from biogas and electrochemical reduction of carbon dioxide. And a Once you have ethylene, you are able to transform it into countless products used around the world.
The next step is to determine the suitability of the catalyst for industrial-scale production while trying to further increase the yield. But for now, finally improving a method that has remained an unfulfilled promise since the 1980s marks an important milestone.
“The complexity of the system and the dynamics that occur, it’s almost like art,” says Mosevitzky Lis. “The catalyst core and shell are subjected to very extreme processes, generating all kinds of interesting things on the surface. It’s beautiful.”
More information:
Kun Zhao et al, Rare earth mixed oxides promoted by lithium carbonate as a widespread strategy for oxidative coupling of methane with exceptional yields, Natural communications (2023). DOI: 10.1038/s41467-023-43682-5
Provided by Lehigh University
Quote: Core-shell “chemical loop” increases efficiency of greener approach to ethylene production (January 12, 2024) retrieved January 12, 2024 from
This document is subject to copyright. Except for fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.