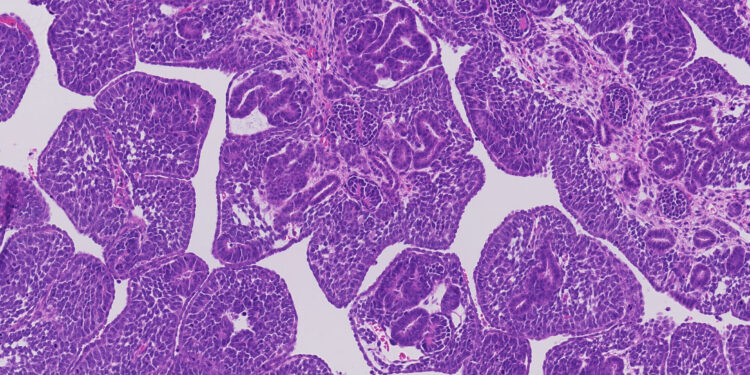
Histology characteristic of a newborn Wilms tumor driven by a Foxr2 mutation. Credit: Ronald de Krijger / Princess Máxima Center for Pediatric Oncology
Researchers have discovered that certain childhood cancers have a significantly higher number of DNA changes than we thought previously, modifying the way we consider the tumors of children and possibly opening up new or reused treatment options.
Focusing on a type of child’s kidney cancer, known as Wilms Tumor, an international team genetically sequenced several tumors with a resolution that was not possible before.
This collaboration included researchers from the Wellcoma Sanger Institute, the University of Cambridge, Princess Máxima Center for Pediatric Oncology, Oncode Institute in the Netherlands, Great Ormond Street Hospital and Cambridge University Hospital Nhs Foundation Trust.
They discovered many more genetic changes by cancer cell than expected, adding millions of changes per tumor. This suggests that some infant tumors could be eligible for treatments such as immunotherapy.
In the study, published in Nature communicationsThe team also describes a single spontaneous genetic change which causes a rare type of Wilms tumor, whose children are born, and that this change occurs early during development in the uterus.
They found that these tumors have a particular appearance under the microscope and the genetic profile, which implies that it may be possible, in the future, to develop a personalized therapy and clinical plans to help those who have this genetic change.
This research questions the widespread idea that childhood cancers have a very small number of genetic changes and suggests rather that there could be effective adult treatments that could be adapted to infant tumors in the future.
Wilms’s tumor is a type of kidney cancer that largely affects children under the age of five. In the United Kingdom, around 85 children receive a diagnosis of Wilms tumor each year.
Previously, we thought that infantile cancer tumors, like those of Wilms tumor, had a small number of genetic changes, also called genetic variants.
To study how and why these tumors are presented so early in life, the Sanger Institute team and their collaborators applied the latest genomic sequencing techniques to better understand how and when these genetic changes occurred.
The entirely genome sequencing methods allow researchers to find genetic changes shared by all the cells of the tumor. Although this can work well for adult tumors, because cells have had more time to develop, infant tumors have fewer shared genetic changes, which means that the large number of mutations that are not shared by all cells are missed.
To overcome this, the team used two cutting -edge techniques: nanorate sequencing, otherwise known as Nanoseq, and sequencing the whole genome of organoids derived from a cell to study renal tumors with much higher resolution. These methods allow scientists to find genetic changes that could be present in a single cell of cancer.
The team used these methods to genetically sequence the samples of Wilms tumors of four children, six months old. They found that a single cancer cell had 72 to 111 additional genetic changes in addition to those already identified via sequencing methods for the whole genome in bulk.
This means that when the overall number of cells in the tumor is taken into account, there are most likely millions of genetic changes per tumor overall, not the low numbers that were previously thought.
In addition to the modification of our understanding of infant tumors, this new observation could also have implications for treatment. Researchers suggest that with this number of possible genetic changes, it is likely that tumors could become treatment resistant faster, or that certain drugs may not work at all.
However, this discovery could also mean that infant tumors are better candidates for existing treatments that are currently used for adult tumors, such as immunotherapy.
The team also traced the evolution of tumors in three children and discovered a new mutation that causes Wilms tumor. This only change in the Foxr2 gene has proven to occur while the kidney developed in the uterus and is associated with a particular appearance of the tumor under the microscope and a specific set of RNA changes.
Researchers suggest that this could be used to identify these tumors and that one day it may be possible to develop specific personalized treatment for certain genetic profiles in Wilms tumor.
Dr. Henry Lee-Six, co-first author of the Wellcoma Sanger Institute, said: “Generalized sequencing methods are incredibly useful for a large number of cancer tumors, especially in adults. However, they fail to capture the real genetic complexity of cancers, in particular those who occur in young children.
“With these latest genomic sequencing techniques, we can now see a much more detailed image of the Wilms tumor, which can occur in newborns.
Dr. Jarno Drost, co-ennior author at the Princess Máxima Center for Pediatric Oncology and the Oncode Institute in the Netherlands, said: “Being able to trace the evolution of a tumor can discover crucial information on how and why it is developing.
“The treatment of the Wilms tumor must carefully balance the treatment of the tumor and the reduction in the risk of recurrence, while minimizing the impact that this can have on the quality of life of a young child and his family. By understanding the genetic changes that cause tumors, and in this case, identifying different genetic subsets, this could lead to more targeted treatment options.”
Professor Sam Behjati, author Co-Senior at the Wellcoma Sanger Institute and Cambridge University Hospitals NHS Foundation Trust, said: “It was a much considered belief that infantile tumors had a much lower number of genetic changes than adult tumors. However, thanks to the development of new genomic sequencing tools, we were able to show that, at least in these cases This is not true.
“Our results suggest that infantile tumors have at least four times more genetic changes per cell than expected, adding millions of changes per tumor, stressing that what we could see before was just the tip of the iceberg.
“This has implications for child’s kidney cancer and maybe other infant tumors. If we fully understand child cancer, we can develop new ways to treat them or reuse existing treatments to obtain options to those who need it as quickly as possible.”
More information:
H. Lee-Six, et al. High resolution clonal architecture of hypomuted Wilms tumors, Nature communications (2025). DOI: 10.1038 / S41467-025-59854-4
Supplied by Wellcome Trust Sanger Institute
Quote: Child’s kidney cancer has millions of genetic changes, opening the door to possible treatments (2025, May 29) recovered on May 29, 2025
This document is subject to copyright. In addition to any fair program for private or research purposes, no part can be reproduced without written authorization. The content is provided only for information purposes.