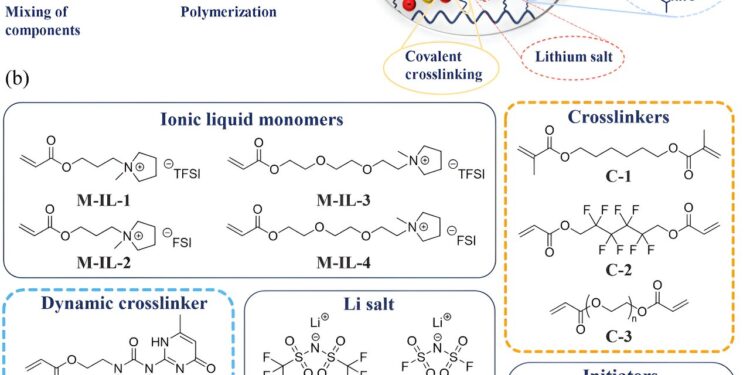
a) Schematic representation of the preparation of the gel electrolyte and the characteristics highlighted, b) chemical structures of the gel components. Credit: Advanced functional materials (2024). DOI: 10.1002/adfm.202403487
A new type of gel, developed by chemists at Martin Luther University Halle-Wittenberg (MLU), could help make lithium-ion batteries safer and more powerful. The gel is designed to prevent leakage of the highly flammable electrolyte liquid.
Early laboratory studies show that it also improves battery performance and life. The researchers published their work in the journal Advanced functional materials.
Lithium-ion batteries are real powerhouses. “They charge faster than conventional rechargeable batteries and can therefore be used in almost all areas of everyday life,” says Professor Wolfgang Binder, head of the Macromolecular Chemistry research group at MLU.
“However, electrolytes, which carry the ions that conduct current between the electrodes, are highly flammable. This can cause the battery to catch fire or explode if damaged.”
Researchers at the University of Munich are working to improve the safety of lithium-ion batteries. “We have developed a polymer that can be introduced into the battery cell. The electrolyte is bound to this substance, but the ions can continue to flow freely between the electrodes,” explains Dr. Anja Marinow, a chemist at the University of Munich.
“The filling has a gel-like consistency and combines the high conductivity of liquids with the thermal stability and robustness of polymers.”
Gel batteries with traditional electrolytes are not new in themselves: they are used, for example, as starter batteries for motorcycles. However, when combined with lithium ions, they represent unexplored technological territory.
This is largely due to a particular challenge. “In conventional lithium-ion batteries, liquid electrolytes form a stabilizing layer on the electrodes when the battery is first charged. This is crucial for the battery’s performance and service life,” explains Marinow.
“However, we needed a fundamentally new design for gel electrolytes.” The researchers solved this problem by integrating an ionic scaffold into the polymer’s molecular chains.
Dr. Anja Marinow and Professor Wolfgang Binder present the filling gel they developed for lithium-ion batteries. This gel should make batteries safer and more efficient. Credit: University of Halle / Heiko Rebsch
Initial laboratory tests show that this approach could increase battery safety and even improve their service life and performance. “A voltage of around 3.6 volts is considered a critical value for electrolyte stability in conventional lithium-ion cells,” says Binder. “Our gel electrolytes remain stable even at over 5 volts.”
Sustainability is also a priority: the gels are designed so that they can be recycled relatively easily in the event of a defect or at the end of the battery’s service life. However, extensive long-term studies still need to be conducted before the new lithium gel batteries can be produced on an industrial scale.
The research work was carried out within the framework of the “BAT4EVER” project. Universities, research centres and industrial partners from Germany, Belgium, Luxembourg, Italy, Spain and Turkey participated in the project.
The research, particularly in the field of sustainability, is planned to be continued and expanded within the framework of the “European Centre for Just Transition and Impact Transfer Research (JTC)”. The centre, which is currently being set up at the MLU, will develop research-based solutions that support structural changes in Saxony-Anhalt, for example in the field of the circular economy or social innovation.
More information:
Zviadi Katcharava et al., Design of conductive pyrrolidinium-based dual-network gel electrolytes: performance tailoring through dynamic and covalent crosslinking, Advanced functional materials (2024). DOI: 10.1002/adfm.202403487
Provided by Martin Luther University Halle-Wittenberg
Quote: Chemists create gel to prevent leaks and increase the life of lithium-ion batteries (2024, September 2) retrieved September 2, 2024 from
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without written permission. The content is provided for informational purposes only.