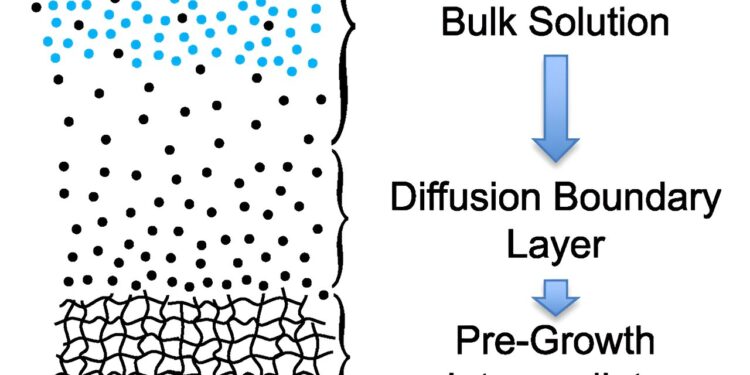
Graphical summary. Credit: Matter (2024). DOI: 10.1016/j.matt.2024.08.011
Remember that old high school chemistry experiment where salt crystals precipitate from a salt water solution – or maybe the one where candy crystals form from sugar water? It turns out that your understanding of how crystals form in these solutions might be wrong.
A new theory demystifies the crystallization process and shows that the material that crystallizes is the dominant component of a solution, namely the solvent, not the solute. This theory could have implications for everything from drug development to understanding climate change.
The article is published in the journal Matter.
“Crystals are ubiquitous—we use them in everything from technology to medicine—but our real understanding of the crystallization process is lacking,” says James Martin, a professor of chemistry at North Carolina State University and author of an article in Matter which describes the theories.
“The prevailing ideas about dissolution and precipitation are that they are essentially the inverse of each other, but that is not the case. In reality, they are completely different processes,” says Martin.
“Taking as an example the high school chemistry experiment of extracting a precipitate from a solution: when I dissolve salt (the solute) in water (the solvent), water is dominant. It dissolves the salt by basically tearing it apart,” Martin says. “If I then want to grow a salt crystal from this solution, the dominant phase must become the salt, which is the solvent at that point and the one that forms the crystal.”
Thermodynamic phase diagrams, which describe concentration- and temperature-dependent transition points in solutions, can be used to illustrate the new theory, called transition zone theory.
The theory demonstrates that crystallization occurs in two stages: First, a melt-like pre-growth intermediate forms. Then, this intermediate can organize itself into a crystal structure.
“To grow a crystal from a solution, you need to quickly separate the solvent and solute,” Martin explains. “When we talk about ‘melting’ here, we are talking about the pure solvent phase before the crystals form. The difference here is that my theory shows that you get better and faster crystal growth by moving your solution to conditions that emphasize fusion. the solvent; in other words, the solvent – not the impurity it contains – controls the rate of crystal growth.
Martin applied his theory to a number of different solutions, concentrations and temperature conditions and found that it accurately described the rate and size of crystal formation.
“The main problem with previous descriptions of crystallization was the perception that crystals grow by diffusing independent solute particles and then attaching them to a growing crystal interface,” says Martin. “Instead, it is necessary to understand the cooperative ensembles of the solvent to describe crystal growth.”
According to Martin, the important aspect of the new theory is its focus on understanding how impurities in the solute disrupt this cooperative set of solvents.
“By understanding the interaction of temperature and concentration, we can predict exactly how quickly and to what size crystals will grow out of solution.”
Martin believes that phase diagrams could have important applications not only for crystal formation, but also for preventing crystal formation, for example to prevent the growth of kidney stones.
“Crystals are the basis of technology: they are everywhere around us and impact our daily lives,” explains Martin. “This theory gives researchers simple tools to understand the ‘magic’ of crystal growth and make better predictions. It is an example of how fundamental science lays the foundation for solving all kinds of problems in the real world.”
More information:
James D. Martin, Solutes do not crystallize! Information from phase diagrams demystifies the “magic” of crystallization, Matter (2024). DOI: 10.1016/j.matt.2024.08.011
Provided by North Carolina State University
Quote: Chemist challenges traditional views on crystal growth (October 2, 2024) retrieved October 2, 2024 from
This document is subject to copyright. Except for fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for informational purposes only.