Credit: ACS catalysis (2024). DOI: 10.1021/acscatal.4c03521
Researchers from the Department of Chemical Engineering at the University of Twente, led by Georgios Katsoukis, have discovered how the chemical environment around copper electrodes can significantly influence the conversion of carbon dioxide (CO₂) to formate. This discovery may help improve the selectivity of CO₂ reduction reactions, providing new insights into how to control these processes more effectively.
One way to create a more sustainable and circular economy is to capture CO2 emissions and transform them into useful resources. But to do this, these CO2 reduction technologies must be optimized and much more efficient.
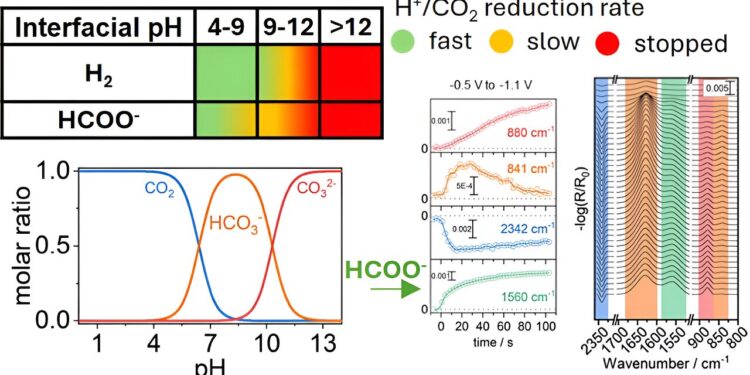
In this study, published in ACS catalysisThe research team studied how CO₂ reacts on the surface of copper electrodes in an aqueous environment. By changing the pH near the electrode, the team discovered that the local chemical environment is crucial in determining how quickly and efficiently CO₂ can be converted to formate, a useful chemical with many industrial applications .
Selectivity in CO₂ reduction reactions has been a long-standing challenge because multiple products can form depending on the reaction conditions. This discovery challenges the traditional focus solely on catalyst material and highlights the importance of optimizing surrounding chemical conditions.
This research highlights the importance of controlling the chemical environment of CO2 reduction process to improve selectivity and efficiency. By finely tuning the conditions around the copper electrode it may be possible to improve the selectivity towards desired products such as formate.
At the same time, it could also extend the life of the electrode. These advances could play a crucial role in developing more efficient carbon dioxide conversion systems.
The results of this study provide a model for future research and development of CO₂ reduction technologies. By focusing on optimizing the chemical environment, in addition to the catalyst, scientists can work to create more selective and efficient systems.
This approach brings us closer to practical solutions to transform CO₂ emissions into useful resources, thereby promoting a more sustainable and circular economy.
More information:
Georgios Katsoukis et al, Time-resolved infrared spectroscopic evidence for pH-dependent interfacial kinetics of formate evolution on Cu electrodes, ACS catalysis (2024). DOI: 10.1021/acscatal.4c03521
Provided by the University of Twente
Quote: Chemical engineers provide new insights into converting CO₂ with electricity (September 27, 2024) retrieved September 27, 2024 from
This document is subject to copyright. Except for fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for informational purposes only.