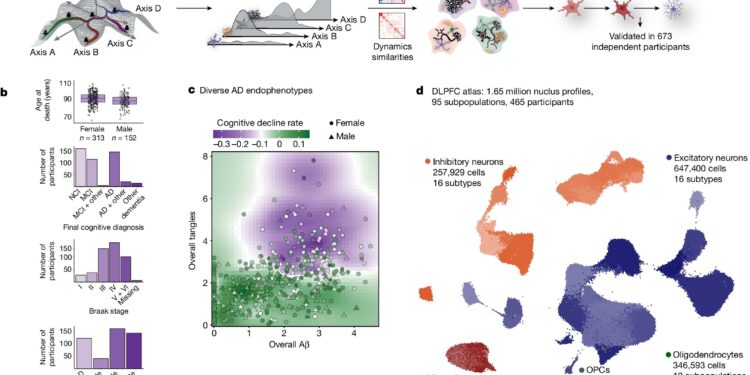
Cellular atlas of the aged human DLPFC in older adults. Credit: Nature (2024). DOI: 10.1038/s41586-024-07871-6
An analysis of more than 1.6 million brain cells from older adults has detected cellular changes that occur in the early stages of Alzheimer’s disease, potentially revealing new ways to prevent the most common cause of dementia in older adults.
The study also identified a second community of cells that pushes the aging brain onto a different pathway that does not lead to Alzheimer’s disease.
“Our study underscores that Alzheimer’s is a disease involving many cells and their interactions, not just one type of dysfunctional cell,” says Columbia neurologist Philip De Jager, who led the study with Vilas Menon, assistant professor of neurological sciences at Columbia University Vagelos College of Physicians and Surgeons, and Naomi Habib of the Hebrew University of Jerusalem.
“We may need to modify cellular communities to preserve cognitive function, and our study reveals points along the sequence of events leading to Alzheimer’s disease where we may be able to intervene.”
The research is published in the journal Nature.
Analysis of data from 1.6 million brain cells
The study was a technical marvel, cleverly combining new molecular technologies, machine learning techniques and a vast collection of brains donated by aging adults.
Although previous studies of brain samples from Alzheimer’s patients have provided some insight into the molecules involved in the disease, they have not revealed much detail about the role these genes play in the long sequence of events leading to Alzheimer’s and the cells involved at each step of the process.
“Previous studies analyzed whole brain samples and lost all the cellular details,” De Jager says. “We now have tools to look at the brain at finer resolution, down to the level of individual cells. When we combine this with detailed information about the cognitive state of brain donors before they died, we can reconstruct trajectories of brain aging from the earliest stages of the disease.”
The new analysis involved more than 400 brains, provided by the Religious Orders Study and the Memory & Aging Project based at Rush University in Chicago.
In each brain, the researchers took several thousand cells from a brain region affected by Alzheimer’s disease and aging. Each cell was then subjected to a single-cell RNA sequencing process, which allowed them to learn about the cell’s activity and determine which genes were active.
Data from 1.6 million cells were then analyzed by algorithms and machine learning techniques developed by Menon and Habib to identify the types of cells present in the sample and their interactions with other cells.
“These methods have allowed us to gain new insights into the potential sequences of molecular events that lead to impaired brain function and cognitive impairment,” Menon says. “This was only possible because of the large number of brain and cell donors from which the team was fortunate to generate data.”
Aging and Alzheimer’s disease
Because the brains came from people at different stages of the disease process, the researchers were able to solve a major challenge in Alzheimer’s research: identifying the sequence of changes in cells involved in Alzheimer’s disease and distinguishing those changes from those associated with normal brain aging.
“We propose that two different types of microglial cells – the brain’s immune cells – initiate the process of amyloid and tau accumulation that defines Alzheimer’s disease,” says De Jager.
Then, once the pathology has accumulated, different cells called astrocytes play a key role in altering the electrical connectivity of the brain, leading to cognitive impairment. The cells communicate with each other and bring in additional cell types that lead to a profound disruption in the functioning of the human brain.
“These are exciting new insights that can guide innovative therapeutic development for Alzheimer’s disease and brain aging,” says De Jager.
“By understanding how individual cells contribute to different stages of the disease, we will know the best approach to reduce the activity of pathogenic cell communities in each individual, returning brain cells to their healthy state,” says De Jager.
More information:
Naomi Habib, Cellular communities reveal trajectories of brain aging and Alzheimer’s disease, Nature (2024). DOI: 10.1038/s41586-024-07871-6. www.nature.com/articles/s41586-024-07871-6
Provided by Columbia University Irving Medical Center
Quote:A cell community in the brain drives Alzheimer’s disease, large-scale analysis reveals (2024, August 28) retrieved August 28, 2024 from
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without written permission. The content is provided for informational purposes only.