Graphical summary. Credit: Cellular chemical biology (2024). DOI: 10.1016/j.chambiol.2024.01.007
Once entering the body, drugs, in addition to fulfilling their therapeutic function, are biochemically transformed by the action of the metabolic machinery, a process which facilitates their expulsion. This biotransformation leads to a gradual disappearance of the drug, which is transformed into its metabolites.
These, in turn, can reach high concentrations in the body and also exhibit biological activity that may be different from that of the original drug. In other words, the metabolites and the drug coexist in the body and can cause effects different from those obtained with the individual molecules.
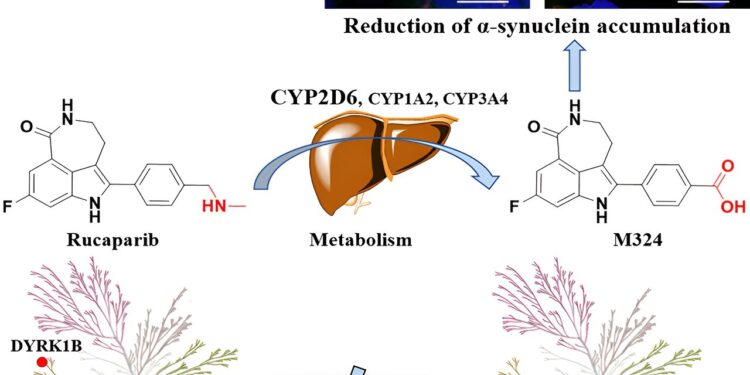
This is the case of Rucaparib, a drug used in chemotherapy against ovarian, breast and, more recently, prostate cancer, and its metabolite, the molecule M324. Rucaparib is part of a group of drugs designed to treat several types of cancers with alterations in DNA repair. More precisely, they are inhibitors of the PARP1 enzyme, involved precisely in the process of repairing mutations in genetic material.
A study led by researchers Albert A. Antolin, from the Oncobell program of the Bellvitge Biomedical Research Institute (IDIBELL) and ProCure from the Catalan Institute of Oncology (ICO), and Amadeu Llebaria, from the Institute of Advanced Chemistry of Catalonia (IQAC-CSIC), showed that Rucaparib and its main metabolite M324 present differential activities.
Published in the journal Cellular chemical biology, the paper analyzed Rucaparib and M324, making a computational prediction of the metabolite’s activity. The article describes the synthesis of M324 and its bioassay, demonstrating that the drug and its metabolite have differentiated activities and act synergistically in certain prostate cancer cell lines.
Surprisingly, M324 reduced the accumulation of the protein α-synuclein (an important component of Lewy bodies) in neurons derived from patients with Parkinson’s disease, a neurodegenerative disease characterized by a movement disorder and in which neurons do not produce sufficient amounts of the neurotransmitter dopamine. .
Specifically, the synergy demonstrated between Rucaparib and M324 in prostate cancer cell lines could have an impact on clinical trials for advanced stages of this type of cancer. On the other hand, the fact that M324 is able to reduce the abnormal accumulation of α-synuclein in neurons derived from stem cells of a patient with Parkinson’s disease highlights the therapeutic potential of this metabolite and its possible pharmacological application for the treatment of this neurodegenerative disease.
These results were obtained thanks to the collaboration of the IDIBELL and ICO groups led by Miquel Àngel Pujana and Álvaro Aytés, and the group of Antonella Consiglio, IDIBELL and UB.
The researchers used computational and experimental methods to comprehensively characterize the pharmacology of the M324 molecule for the first time. The first author of the work, Huabin Hu, made a comprehensive prediction of the differential activity of the original drug and its product, resulting in different spectra of the cellular protein phosphorylation pattern.
Carme Serra, from the MCS group at IQAC-CSIC, synthesized the metabolite M324, which allowed experimental verification of the computational prediction in biological and cellular analyses. The results obtained could have implications for clinical treatment with Rucaparib and, in turn, open new opportunities for drug discovery.
In summary, the study paves the way for a new conceptual perspective in pharmacology: one that views drug metabolism not as an undesirable process that degrades and eliminates the therapeutic molecule from the body, but rather as a process that can present potential benefits from a therapeutic point of view. Indeed, the work highlights the importance of characterizing the activity of drug metabolites to globally understand their clinical response and apply it to precision medicine.
More information:
Huabin Hu et al, Identification of the differential biological activity and synergy between the PARP inhibitor rucaparib and its main metabolite, Cellular chemical biology (2024). DOI: 10.1016/j.chambiol.2024.01.007
Provided by the Spanish National Research Council
Quote: Anticancer drug opens new avenue for treating Parkinson’s disease (February 9, 2024) retrieved February 9, 2024 from
This document is subject to copyright. Apart from fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.