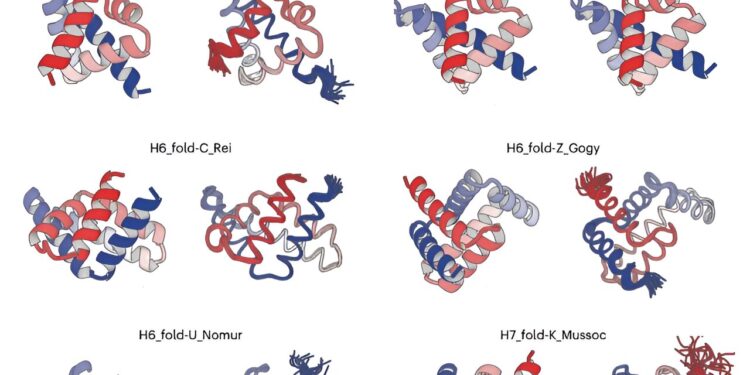
Credit: Nature Structural and molecular biology (2024)
A team of researchers has developed an innovative method to engineer fully α complex proteins, characterized by their non-uniformly arranged α helices, as seen in hemoglobin. Using their new approach, the team successfully created five unique all-α protein structures, each distinguished by their complex arrangements of α-helices. This ability holds immense potential in the design of functional proteins.
This research was published in the journal Nature Structural and molecular biology.
Proteins fold into unique three-dimensional structures based on their amino acid sequences, which then dictate their function. Although significant progress has been made in de novo protein design, the ability to design fully α complex proteins, in which α helices are not arranged in parallel within three-dimensional structures, has been lacking.
“Artificially designed proteins mostly have simple structures, but nature presents us with complicated ‘designs,'” said Professor Nobuyasu Koga of the Center for Exploratory Research on Life and Living Systems (ExCELLS) at the National Institutes of Health. natural sciences (NINS). This gap led the team to search for a method to design such complex all-α proteins.
The team began by examining structures deposited in the Protein Data Bank (PDB) and identified 18 typical helix-loop-helix motifs. They then demonstrated that a broad spectrum of all-α protein tertiary structures, ranging from simple to complicated, can be computationally generated by combining these identified typical motifs and canonical α-helices.
“It is surprising that such a diverse set of fully α protein structures can be generated simply by combining typical or canonical components of natural proteins,” said Dr. Koya Sakuma, a former Ph.D. student at SOKENDAI (Higher University of Advanced Studies).
The team selected five unique all-α proteins, each with five or six α-helices and distinguished by their complex spatial arrangements, from the computer-generated structures. They then performed a de novo design of amino acid sequences capable of folding into the five selected all-α protein structures.
The experimental results were remarkable. “The structures resulting from the structure determination process have complex shapes, which perfectly match the computer-designed structures,” said Dr. Naohiro Kobayashi, principal investigator at RIKEN.
Thanks to this developed method, it is now possible to create fully α proteins with complex shapes. The functions of proteins depend intrinsically on their three-dimensional structures.
If we can design proteins with complex shapes, this increases the potential for building functional sites within them. This groundbreaking research will pave the way for the creation of new functional proteins, which will contribute to healthcare and life sciences.
More information:
Design of complex structures of all-α proteins, Nature Structural and molecular biology (2024). DOI: 10.1038/s41594-023-01147-9 www.nature.com/articles/s41594-023-01147-9
Provided by the National Institutes of Natural Sciences
Quote: Breakthrough in the design of complex structures of all-α proteins (January 4, 2024) retrieved January 4, 2024 from
This document is subject to copyright. Except for fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.