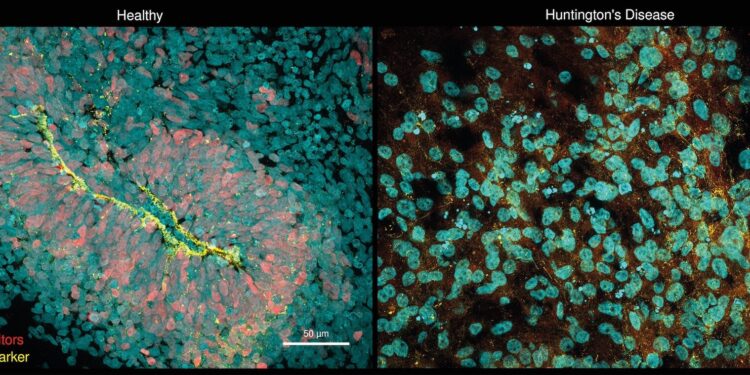
Huntington’s disease organoids (right) show almost no neuronal progenitors (red) and also show cell polarity defects (yellow). These defects have been described in the literature in human fetuses with Huntington’s disease. Credit: Selene Lickefett, Heinrich-Heine-Universität Düsseldorf, Werner Dykstra, Max Delbrück Center
For the first time, researchers have identified the CHCHD2 gene as being involved in Huntington’s disease (HD), an incurable genetic neurodegenerative disease, and have identified this gene as a potential new therapeutic target. In a brain organoid model of the disease, the researchers found that mutations in the Huntington’s HTT gene also affect CHCHD2, which is involved in maintaining normal mitochondrial function.
The study was published in Nature Communications.
Six different laboratories of the Max Delbrück Center participated in the study, led by Dr. Jakob Metzger from the Laboratory of Quantitative Stem Cell Biology and the Laboratory of Stem Cell Metabolism of Prof. Alessandro Prigione at the Heinrich Heine University Düsseldorf (HHU). Each laboratory contributed its unique expertise on Huntington’s disease, brain organoids, stem cell research and genome editing.
“We were surprised to discover that Huntington’s disease can impair early brain development through defects associated with mitochondrial dysfunction,” says co-senior author Dr. Pawel Lisowski from the Metzger lab at the Max Delbrück Center.
Additionally, “the organoid model suggests that HTT mutations damage brain development even before clinical symptoms appear, highlighting the importance of early detection of late-onset neurodegenerative disease,” adds Selene Lickfett, co-senior author and PhD student in the School of Mathematics and Natural Sciences in Prigione’s lab at HHU.
The unusual repetition of three letters
An organoid is a three-dimensional, organ-like structure that researchers grow in the lab from stem cells. Depending on the disease and research question, organoids can be grown from different types of tissue. Measuring just a few millimeters, they serve as a model for how different cell types interact. No other lab model provides such a detailed look at cell function in the human body.
Huntington’s disease is caused by the excessive repetition of the nucleotides cytosine, adenine and guanine in the Huntington’s gene HTT. People with 35 or fewer repeats are generally not at risk for the disease, while those with 36 or more have been linked to the disease. The higher the number of repeats, the earlier the symptoms of the disease are likely to appear, said Metzger, one of the study’s senior authors. The mutations cause nerve cells in the brain to gradually die.
Affected people gradually lose control of their muscles and develop psychiatric symptoms such as impulsivity, delusions and hallucinations. Huntington’s disease affects about five to ten people in 100,000 worldwide. Existing therapies only treat the symptoms of the disease; they do not slow its progression or cure it.
The challenge of HTT gene editing
To study the effect of HTT gene mutations on early brain development, Lisowski first used variations of Cas9 gene editing technology and manipulation of DNA repair pathways to modify healthy induced pluripotent stem cells so that they carried large numbers of CAG repeats. This presented a technical challenge because gene editing tools are not effective in genetic regions that contain sequence repeats, such as the CAG repeats in HTT, Lisowski says.
The genetically modified stem cells were then grown to form brain organoids, three-dimensional structures that resemble early-stage human brains. When the researchers analyzed the gene expression profiles of the organoids at different stages of development, they noticed that the CHCHD2 gene was consistently underexpressed, which reduced the metabolism of the neuronal cells.
CHCHD2 is involved in the health of mitochondria, the energy-producing structures of cells. CHCHD2 has been implicated in Parkinson’s disease, but never before in Huntington’s disease.
They also found that when they restored the function of the CHCHD2 gene, they could reverse the effect on neuronal cells. “That’s surprising,” Lickfett says. “It suggests in principle that this gene could be a target for future therapies.”
Moreover, defects in neural progenitor cells and brain organoids occurred before potentially toxic aggregates of mutated huntingtin protein developed, Metzger adds, indicating that disease pathology in the brain may begin well before it is clinically evident.
“The most common idea is that the disease progresses through degeneration of mature neurons,” Prigione says. “But if changes are occurring in the brain at a very young age, therapeutic strategies may need to focus on much earlier time points.”
Far-reaching implications
“Our genome editing strategies, particularly the deletion of the CAG repeat region in the Huntington gene, have shown great promise in reversing some of the developmental defects that we have observed. This suggests a potential gene therapy approach,” Prigione says. Another potential approach could be therapies aimed at increasing CHCHD2 gene expression, he adds.
These findings could also have broader applications for other neurodegenerative diseases, Prigione adds. “Early treatments that reverse the mitochondrial phenotypes shown here could be a promising avenue for combating age-related diseases like Huntington’s disease.”
More information:
Lisowski, P. et al. Mutant huntingtin impairs neurodevelopment of human brain organoids through CHCHD2-mediated neurometabolic failure, Nature Communications (2024). DOI: 10.1038/s41467-024-51216-w. www.nature.com/articles/s41467-024-51216-w
Provided by the Max Delbrück Center for Molecular Medicine
Quote:A new culprit in Huntington’s disease: Brain organoid model implicates gene in disease progression (2024, August 22) retrieved August 22, 2024 from
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without written permission. The content is provided for informational purposes only.