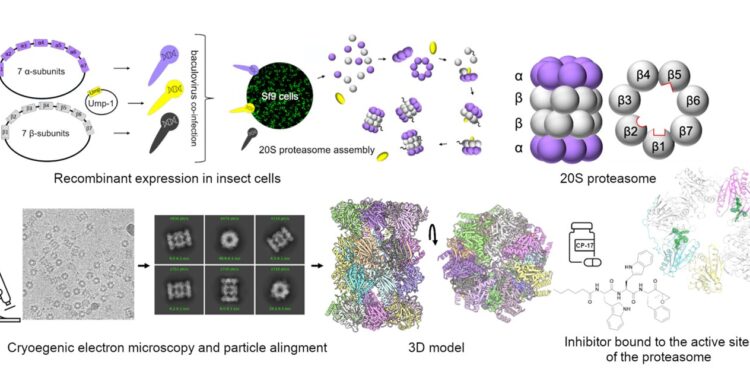
Recombinant proteasome of the parasite Trichomonas vaginalis, successfully produced in insect cells. The structure of the complex, composed of 28 subunits, was analyzed by electron cryomicroscopy. Inhibitors linked to the catalytic site of the proteasome block its function and thus serve as potential leads for new therapies. Credit: IOCB Prague
Researchers at IOCB Prague are deepening the understanding of how drugs work and what needs to be done to develop their most effective variants. In an ongoing study, they focused on the disease caused by the protozoan parasite Trichomonas vaginalis, particularly because of the recent emergence of strains resistant to conventional treatments.
With the aim of finding a new weak point of this parasite, the research group led by Dr. Evžen Bouřa succeeded in preparing a key enzyme complex: the proteasome. This provided essential knowledge for the development of new, effective drugs.
The research was carried out in collaboration with colleagues at the University of California, San Diego. An article presenting the work was published in the journal Natural communications.
The proteasome is a complex protease that functions as a recycling device for old proteins in cells. It is a cylindrical enzyme complex composed of seven subunits arranged in four superimposed circles. When old proteins enter this cylinder, they are broken down into small pieces that are later used by the cell to make new proteins.
If it doesn’t work properly, old proteins build up, causing the cell to die. This is why blocking proteasome activity is used, for example, against certain types of malignant tumors.
Likewise, it should also be possible to treat trichomoniasis, a very common sexually transmitted infection, which increases the risk of contracting HIV. The objective is therefore to identify the substances which block the functioning of the proteasome inside Trichomonas vaginalis cells.
However, since the proteasome of this unicellular organism has never been satisfactorily isolated, the research group at IOCB Prague prepared an artificial proteasome identical to the natural proteasome. Interestingly, they used insect cells to do this.
This part of their research, on which the success of the entire project depended, turned out to be the most complicated. The fact that the team succeeded in describing the previously unknown structure of the parasite’s proteasome is therefore of crucial importance.
“The proteasome looks the same in every animal or protozoan, but the details of this cylindrical structure differ. It is these details that we need to know in order to create a substance that is toxic to the parasite but not harmful to the human body.
“This is a crucial prerequisite for drug development. Our greatest success is that we were able to prepare a recombinant artificial proteasome that can provide us with the necessary information,” explains Bouřa.
The researchers used advanced electron microscopy, allowing them to see almost every atom. With this ability, they tested the effects of two potential proteasome-inhibiting substances. They saw how they bind to the active site of the proteasome and under what circumstances the proteasome stops functioning, which is fatal to the parasite cell.
More information:
Jan Silhan et al, Structural elucidation of the recombinant Trichomonas vaginalis 20S proteasome linked to covalent inhibitors, Natural communications (2024). DOI: 10.1038/s41467-024-53022-w
Provided by the CAS Institute of Organic Chemistry and Biochemistry
Quote: Artificial proteasome offers insights into new treatments for trichomoniasis (October 8, 2024) retrieved October 8, 2024 from
This document is subject to copyright. Except for fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for informational purposes only.