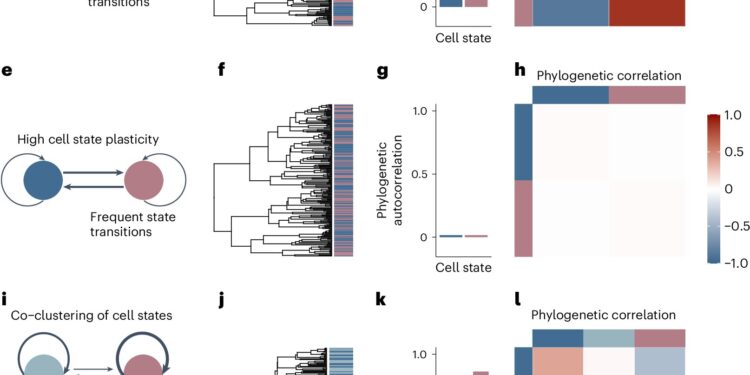
Phylogenetic correlations quantify heritability versus plasticity of single-cell phenotypes. Credit: Genetics of nature (2024). DOI: 10.1038/s41588-024-01920-6
A powerful new analytical tool offers deeper insight into how tumor cells “change shape” to become more aggressive and incurable, according to a study led by researchers at Weill Cornell Medicine and the New York Genome Center.
A tumor cell changes by changing its cell type or state, which alters its basic activity pattern and perhaps even its appearance. This variability or “plasticity” is a hallmark of cancer that leads to diverse tumor cell populations and, ultimately, the emergence of cell types that allow for treatment resistance and metastatic spread.
The new tool, described September 24 in an article published in Genetics of nature, This plasticity can be quantified in tumor cell samples. The researchers demonstrated this by analyzing tumor samples from animal models and human patients, identifying, for example, a key transitional cell state in glioblastoma, the most common form of brain cancer.
“Plasticity is a tremendous driver of cancer spread and resistance to treatment, and we hope this new tool will give us critical insights into these processes – insights we hope to use to more effectively fight cancers,” said the study’s senior author, Dr. Dan Landau, professor of medicine in the division of hematology and medical oncology at Weill Cornell Medicine and a senior faculty member at the New York Genome Center.
The study’s co-first authors, all from the Landau lab, were postdoctoral researcher Dr. Joshua Schiffman, MD/PhD student Andrew D’Avino, and postdoctoral researcher Dr. Tamara Prieto.
Plasticity is normal and widespread in the early stages of life, as cells transition from embryonic stem cells to increasingly differentiated states with highly specialized functions. Some degree of plasticity is also required in mature tissues for repair and maintenance functions. Cancers unfortunately tend to hijack these latent plasticity mechanisms, and cancers with greater plasticity tend to be more difficult to treat successfully.
The researchers called their new tool Phylogenetic Analysis of Trait Heritability, or PATH. For a sample of tumor cells, it quantifies the plasticity of each cell state, based on how often a cell in that state gives rise to progenitor cells that share that state. Cell states that are less likely to be inherited are considered more malleable.
To apply PATH to the analysis of a given cell population, researchers need information about the “lineage,” usually based on DNA markers, showing which cells are descended from the same parent cell. They also need information about individual cell states, which can be defined according to the researcher’s preferences.
“It can be based on the cells’ patterns of genetic activity, their surface receptors, their spatial locations within the tumor, or really anything you can imagine,” Dr. Schiffman said.
The researchers demonstrated PATH in analyses of pancreatic tumors, revealing new details about how these tumors exploit a form of plasticity called epithelial-to-mesenchymal transition, in which epithelial-like cells transform into mesenchymal-like cells, thereby acquiring migratory properties that enable metastatic spread.
“We knew there was a transition with intermediate states, but we didn’t know exactly what was happening,” Dr. Schiffman said. “We were able to provide a clearer picture of these dynamics.”
Similarly, for glioblastoma cells from human patients, PATH-based analysis has shown how tumor cells alternate between more stem-like and mesenchymal states, using a state resembling that of a brain helper cell called an astrocyte as a key intermediate state. Finally, PATH-enhanced profiling of malignant B cells from leukemia patients has revealed an apparent link between certain DNA mutations in leukemia cells and a relatively plastic, stem-like cellular state defined by a key surface receptor.
Overall, the researchers said, PATH provides a very useful new framework for studying tumor development.
Dr. Landau, who is also a member of the Englander Institute for Precision Medicine at Weill Cornell Medicine and the Sandra and Edward Meyer Cancer Center, along with Dr. Schiffman and their colleagues, envision a number of clinical applications for PATH. These include prognostic tests based on the degree of plasticity measured in tumor samples (greater plasticity being a reason to expect greater tumor aggressiveness) and new treatments that would target the more stable, less plastic cellular states of tumors.
The researchers also plan to perform PATH-based analyses of tumor samples before and after different treatments, to determine, for example, which treatments can reduce tumor cell plasticity.
More information:
Joshua S. Schiffman et al., Defining heritability, plasticity, and transition dynamics of cellular phenotypes in somatic evolution, Genetics of nature (2024). DOI: 10.1038/s41588-024-01920-6
Provided by Weill Cornell Medical College
Quote:Analytical tool quantifies cancer’s ability to change shape (2024, September 24) retrieved September 24, 2024 from
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without written permission. The content is provided for informational purposes only.