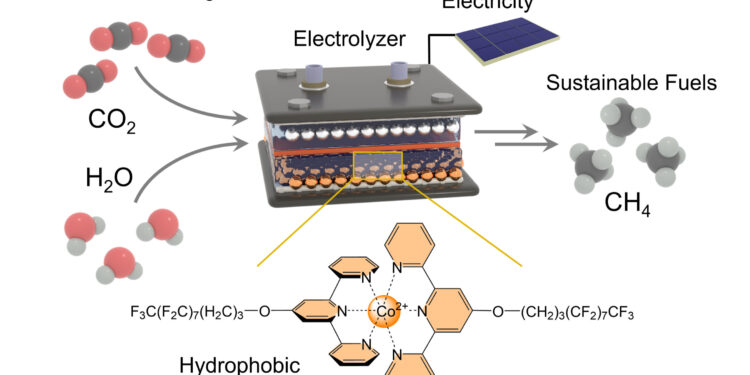
keep the H2O molecules in the electrolyzer (top) away from the active center. At the same time, it removes hydrogen atoms from water molecules and transports them to the active center, where they react with the carbon atom to form methane. Credit: Nikolai Kornienko
Researchers from the University of Bonn and the University of Montreal have developed a new type of catalyst and used it in their study to produce methane from carbon dioxide and water very efficiently using electricity. Methane can be used, for example, to heat apartments or as a raw material in the chemical industry. It is also the main component of natural gas.
However, if it is produced from green electricity, it is largely climate neutral. The knowledge gained from the model system studied by the researchers can be transferred to large-scale technical catalysts. The system could also be used to produce other important chemical compounds. The study was published in the journal Natural chemistry.
Many chemical reactions require energy to start and this energy can be added, for example, by heating the reaction partners or subjecting them to high pressure. “Rather, we used electricity as a motor,” explains Professor Nikolay Kornienko. “By using climate-friendly electricity, we can produce, for example, methane which does not contribute to global warming.”
The researcher recently left the University of Montreal to join the Institute of Inorganic Chemistry at the University of Bonn. He began his final studies while still in Canada and completed them in his new home. “The production of methane, whose chemical formula is CH4“It is a challenge because it is necessary to carry out a reaction between a gas and a liquid,” explains Kornienko.
In this case we are talking about carbon dioxide (CO2) and water (H2O). The researchers used a gas diffusion electrode to bring these two partners together. In the reaction, it is necessary to separate the two oxygen atoms from the carbon atom and replace them with four hydrogen atoms. Hydrogen comes from water.
Preventing side reactions
The problem with this process is that the water would much rather undergo another reaction and would split into hydrogen and oxygen as soon as it was exposed to an electric current. “It’s a competing reaction that we need to avoid,” said Morgan McKee, Kornienko’s assistant, who carried out many of the experiments.
“Otherwise, it would prevent us from producing methane. So we need to prevent water from coming into contact with the electrode. At the same time, we still need water as a reaction partner.”
This is where the newly developed catalyst, deposited on the electrode, comes into play. Above all, this ensures that carbon dioxide reacts more easily and quickly to produce methane. It achieves this thanks to its so-called “active center” which retains carbon dioxide and, in simple terms, also weakens the bonds between the carbon atom and the two oxygen atoms.
These oxygen atoms are then gradually replaced by four hydrogen atoms in the next step. The catalyst needs water at this point in the process. However, he must also keep it at bay to avoid any unwanted side reactions. “To achieve this, we linked long molecular side chains to the active center,” explains Professor Kornienko, who is also a member of the transdisciplinary research area “Matter” at the University of Bonn. “Their chemical structure repels water or, in other words, they are hydrophobic.”
Water-fearing molecular chains
The side chains not only hold the H2O molecules away from the active center and the electrode but they also act as a kind of conveyor belt.
Figuratively speaking, they extract hydrogen atoms from water molecules and transport them to the active center, where they react with the carbon atom. In this way, the CO2 is converted to CH4 in several stages.
This process has an efficiency of over 80% and the reaction produces virtually no unwanted side products. However, the catalyst is not really suitable for large-scale methane production. “The reaction principles we achieved with this catalyst could, however, be applied to other catalytic materials intended for use in large-scale technical applications,” explains Kornienko.
The researcher believes that methane production is not the only area of application of this method. He said it could prove more lucrative in the production of other chemical compounds like ethylene, which is used as a raw material for many plastics.
In the medium term, the new catalytic method could thus be used wherever possible to make plastic production more environmentally friendly.
More information:
Morgan McKee and. al. Hydrophobic molecular assembly at the gas-liquid-solid interface results in highly selective electromethanation of CO2, Natural chemistry (2024). DOI: 10.1038/s41557-024-01650-6
Provided by the University of Bonn
Quote: Electricity-powered catalyst delivers climate-neutral methane production (October 4, 2024) retrieved October 4, 2024 from
This document is subject to copyright. Except for fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for informational purposes only.