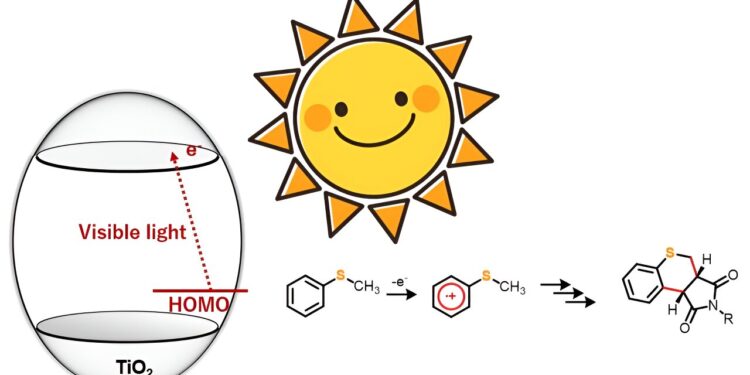
A team of researchers now presents an environmentally friendly and innovative approach for the blue light-enhanced synthesis of heterocyclic thiochromepyrroledione derivatives catalyzed by titanium dioxide. Credit: Professor Yutaka Hitomi, Doshisha University
Heterocyclic compounds are organic molecules with a cyclic structure comprising at least two or more elements. In most cases, these rings are composed of carbon atoms as well as one or more other elements such as nitrogen, oxygen or sulfur. They are highly sought after as raw materials in the chemical and pharmaceutical industry, due to their versatility and excellent physiological activities.
Although several methods are available to synthesize these compounds, most of them involve high temperature and pressure conditions, or the use of precious metal catalysts, which increases the economic and environmental cost of production. heterocyclic organic compounds.
However, a team of researchers from Japan and Bangladesh have proposed a simple but effective method to overcome these challenges. Their study was recently published in the journal Advanced synthesis and catalysis. Using the proposed strategy, the team demonstrated the synthesis of 20 sulfur-containing heterocyclic compounds in the presence of the photocatalyst titanium dioxide (TiO2) and visible light.
The study was led by Professor Yutaka Hitomi of the Department of Applied Chemistry, Graduate School of Science and Engineering, Doshisha University, and co-authored by a Ph.D. candidate Pijush Kanti Roy from Doshisha University, Associate Professor Sayuri Okunaka from Tokyo City University and Dr. Hiromasa Tokudome from TOTO Research Institute Ltd.
TiO2 as a photocatalyst to trigger organic reactions, has attracted the attention of synthetic chemists for some time now. However, many of these processes require ultraviolet light to trigger the reaction. In this study, however, the research team discovered that under anaerobic conditions, organic sulfur compounds such as thioanisole derivatives, when exposed to blue light, react with maleimide derivatives to form bonds carbon-carbon doubles, thus giving a new heterocyclic organic compound.
“We observed that although ultraviolet light generates highly oxidative holes, our approach allows selective one-electron oxidation of substrate molecules using visible light. This approach can thus be used in various chemical reactions organic,” explains Professor Hitomi.
The researchers chose five 4-substituted thioanisoles and four N-substituted maleimides for ring cancellation or ring formation reactions. The team irradiated the starting material with blue light (wavelength > 420 nm) but observed no reaction. However, the introduction of TiO2 in the reaction system led to the synthesis of 20 different thiochromenopyrroledione derivatives in moderate to high yield. They found that within 12 hours of exposure to blue light, the reaction between thioanisole and N-benzylmaleimide led to the formation of a thiochromepyrroledione derivative with a yield of 43%, close to the theoretical maximum yield of 50%.
The research team also observed the substituting effect in the reactions to understand the corresponding mechanistic aspects. From the results, they postulated that the reaction proceeds by charge transfer from the thioanisole to the conduction band of TiO.2. Furthermore, they suggested that blue light irradiation triggered the one-electron oxidation of thioanisole, which further initiated the generation of α-thioalkyl radicals through deprotonation.
In summary, this new and refined approach demonstrates the potential of TiO2 for visible light photocatalysis for organic synthesis. It also provided crucial insights into the chemistry of the synthesis of complex heterocyclic compounds. In the future, this approach may open new possibilities for moving from current resource-intensive industrial chemical processes to a more energy-efficient system.
Professor Hitomi says: “What motivated our study was the desire to contribute to the development of a sustainable chemical industry, and our results appear to be a positive step in this direction.
“We believe that widespread adoption of this visible light-based technology could contribute to accessible and affordable synthesis of pharmaceuticals, with its profound impacts on the health and well-being of millions of people around the world.” Thanks to the efforts of Professor Hitomi and his team, their study has opened new avenues in the field of organic synthesis, with the potential to revolutionize several chemical industries.
More information:
Pijush Kanti Roy et al, Blue light-promoted synthesis of thiochromenopyrroledione derivatives via titanium dioxide-catalyzed carbon-carbon bond formation with thioanisole and maleimide derivatives, Advanced synthesis and catalysis (2023). DOI: 10.1002/adsc.202301021
Provided by Doshisha University
Quote: Chemical synthesis from titanium dioxide: An ecological and innovative approach (January 1, 2024) retrieved on January 2, 2024 from
This document is subject to copyright. Except for fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.