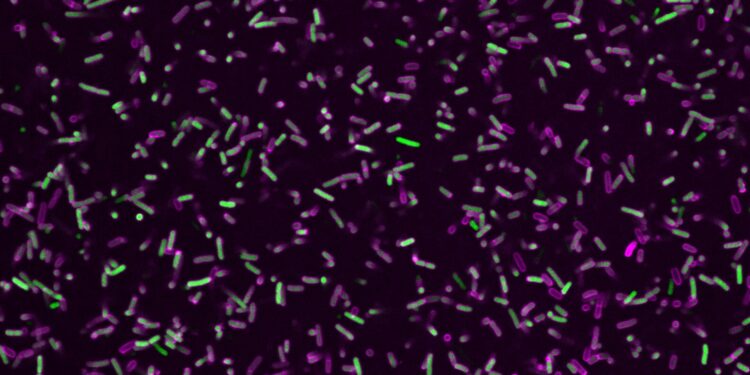
Gram-negative bacterial pathogens (green) surrounded by a protein coat of GBP1 proteins (magenta). Credit: Miriam Kutsch @CAi, Heinrich Heine University Düsseldorf
The human immune system constantly fends off a wide range of invaders, a feat that requires a wide array of cellular troops and molecular weapons. Although much is already known about immune defense cells and the strategies they employ, many molecular details remain elusive.
Today, a research team led by Professor Oliver Daumke, laboratory head at the Max Delbrück Center, has succeeded in elucidating the main mechanism of activation of GBP1, a protein that plays a central role in the fight against certain bacteria.
They report in EMBO magazine on how the protein adopts a particular conformation that allows it to encapsulate invaders and thus neutralize them.
“These results help us to better understand the body’s immune defenses and could, in the future, allow us to stimulate them in a more targeted manner,” explains Daumke.
A destructive protein envelope
GBP1, short for guanylate-binding protein 1, is produced by cells in the body in response to infection. It binds specifically to GTP, a nucleotide and chemical energy transporter in cells, where it orchestrates defense against bacterial pathogens such as Salmonella and Shigella. In some cases, these can cause severe diarrhea.
Not only does GBP1 specifically activate immune defense, but it also forms a protein coat around unwanted intruders. This destroys their membrane, making the bacteria vulnerable and preventing them from multiplying. Scientists already knew about this defense strategy, but the details of how it worked remained unclear, as did the central molecular switch in the GBP1 protein responsible for its activation.
The team used a high-resolution cryoelectron microscope, which allowed them to visualize the three-dimensional structure of the protein, to study each step of this mechanism. “GBP1 is initially present as a single building block. When activated, it unfolds like a Swiss army knife,” explains the paper’s first author, Marius Weismehl, a doctoral student in Daumke’s lab.
“Thousands of these unfolded proteins then assemble into disks, which in turn stack into tubular structures,” continues Weismehl. “These tubes eventually attach to the bacterial membrane, where they reform and wrap around it like a diaper.” In this way, GBP1 proteins neutralize invaders.
The main goal of the study was to uncover the details of how these large protein structures are constructed. “Our microscopy data impressively show how GBP1 proteins stick to membrane surfaces like pins and join together via their heads,” explains Professor Misha Kudryashev. He adds that these new findings constitute a crucial advance in elucidating the mechanistic functioning of GBP1.
“We identified a molecular lever that plays a critical role in the first step of activation,” explains Weismehl. The protein uses the energy stored in GTP to control this lever and activate itself. This mechanism allows the protein to change shape so that it can bind to other dimers and form a stable protein coat around the bacteria. The researchers gained this knowledge by specifically modifying certain sites on the protein, revealing the different functions they perform.
“Our results revealed for the first time the sophisticated activation mechanism of GBP1, which results in the encapsulation of pathogens with an antimicrobial protein coat,” summarizes Daumke.
In the future, according to Weismehl, it will be possible to examine how GBP1 protein assemblies interact with other players in the immune response and thus trigger downstream signaling cascades.
The research team is optimistic that this fundamental knowledge of the human immune system will help better understand bacterial infectious diseases and inform new treatments that specifically improve the immune response during infection.
More information:
Marius Weismehl et al, Structural insights into the activation mechanism of the antimicrobial GBP1, EMBO magazine (2024). DOI: 10.1038/s44318-023-00023-y
Provided by the Max Delbrück Center for Molecular Medicine
Quote: The team reveals the activation mechanism of a protein that fights bacteria (January 24, 2024) retrieved January 25, 2024 from
This document is subject to copyright. Apart from fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.